SYNERGY™ & SYNERGY MEGATRON™
EES PtCr Coronary Stent System
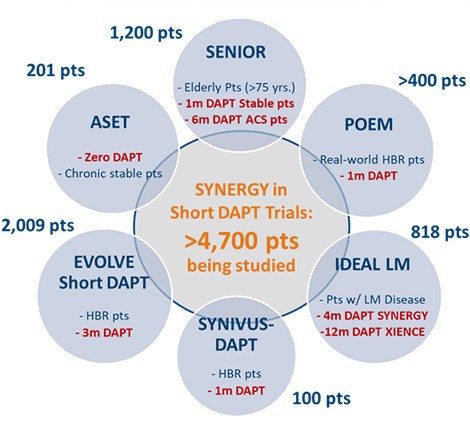
Boston Scientific is continuing to invest in the science to address a significant clinical unmet need with shortened DAPT.
SYNERGY BP-DES was intentionally designed to enable short DAPT
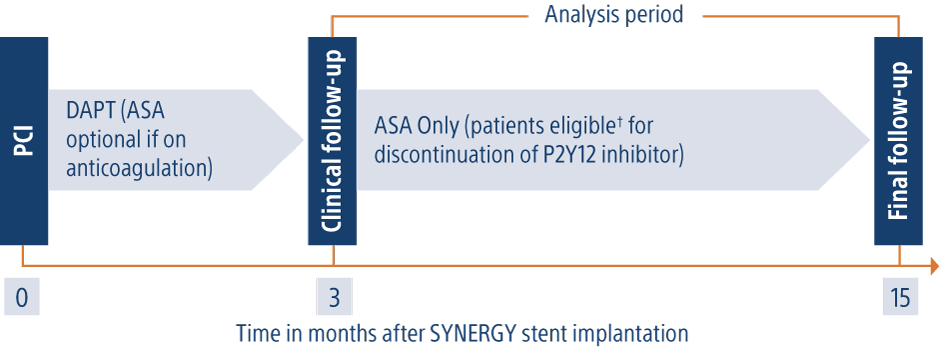
EVOLVE Short DAPT
Purpose
Design
Required clinical follow-up: 3 months, 6 months, 12 months and 15 months post-index procedure.
Endpoints
Secondary Endpoint: Bleeding Academic Research Consortium (BARC) bleeding Type 2, Type 3, and Type 5 between 3-15 months (patients not on chronic anticoagulation).
SENIOR
Purpose
elderly patients undergoing PCI with the SYNERGY™ BP-DES or REBEL™ BMS.
Design
Required clinical follow-up: 1 month, 6 months, 1 year and 2-years post index procedure.
Endpoints
Secondary Endpoint: ARC def/prob ST, BARC - Type 2, Type 3, and Type 5
Progress to Date
12 month primary endpoint data was released at TCT 2017; Data specific to 1-month DAPT cohort was released at EuroPCR 2018. 2-year results were presented at TCT 2018.
ASET
Purpose
Design
N=200 chronic stable angina patients in Brazil
Required clinical follow-up: 1 month, 3 months and 4 months post-index procedure
Endpoints
Primary Bleeding Endpoint: BARC Type 3, Type 5 bleeding at 3 months
Progress to Date
IDEAL LM
Purpose
Patients will undergo standard PCI of the left main coronary artery and will be randomized in a 1:1 fashion to the SYNERGY BP Stent or to the Xience stent. Dual antiplatelet therapy (DAPT) will be stopped at 4 months in the SYNERGY BP Stent arm whereas in the control arm DAPT will be continued for 12 months.
Design
N=818 patients randomized 1:1 to LM PCI with either the SYNERGY BP Stent or the Xience Stent
Required clinical follow-up: 6,12 and 24 months
Endpoints
Secondary Endpoints: Individual components of the primary endpoint; DOCE (cardiac death, MI not clearly attributable to a non-target vessel, and clinically-indicated TLR at 1m, 6m and annually through 3y); ARC Definite/Probable ST; Composite of BARC 3 or 5 bleeding at 24 months
Progress to Date
POEM
Purpose
Design
Endpoints
Secondary Endpoints: Components of primary endpoint, as well as all-cause death, TVR, TLR, major bleeding (BARC 3/5), cerebrovascular event and TLF, at 30 days and 1 year; patient-oriented composite endpoint (composite of any death, any MI, and revascularization) at 30 days and 1 year
Progress to Date
SYNIVUS-DAPT
Purpose
Design
n=100 (U.S.)
Endpoints
Secondary Endpoints: Rate of ARC definite/probable ST, rate of major bleeding, ischemia-driven TLR, ischemia-driven TVR, TLF, TVF, all-cause death and all-cause MI at 1 to 13 months