SYNERGY™ & SYNERGY MEGATRON™
EES PtCr Coronary Stent System
Consistently low sub-acute, late & very late ST in 18,000 patients across 9 studies
| SWEET Registry | Fribourg Experience | Belfast Experience | EVOLVE II Trial | EVOLVE Trial | EVOLVE China | EVOLVE II QCA Study | SCAAR Registry | BIO-RESORT Trial | |
|---|---|---|---|---|---|---|---|---|---|
| N: | 820 | 671 | 185 | 846 | 94 | 205 | 100 | 14,979 | 1172 |
| Acute | 1.5% | 0.3% | 0% | 0.2% | 0% | 0% | 0% | 0.08%* | 0.1% |
| Sub-acute | 0.1% | 0.3% | 0% | 0% | 0% | 0% | 0% | 0.02%* | 0.1% |
| Late | 0.1% | 0.1% | 0% | 0% | 0% | 0% | 0% | 0.2%* | 0.2% |
| Very Late | 0% | 0.3% | 0% |
|
| 0.1%* | 0.3% |
* Cumulative adjusted ARC def ST estimated from Kaplan Meier Curve.
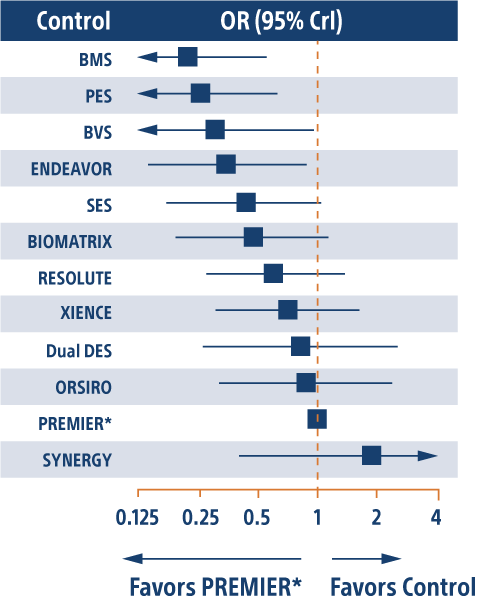
SYNERGY ™ BP Stent Ranked #1 and Promus PREMIER ™ PP Stent Ranked #2 for the lowest relative risk of Definite/Probably Stent Thrombosis
Study Design (ST Analysis)
- 110 prospective, randomized controlled trials included
- 111,088 patients
- Primary endpoint: definite or probable stent thrombosis at 1 year
*All PtCr-EES stents, also includes PROMUS Element and PROMUS Element Plus. Def/prob ST was available in 110 studies with 111,088 patients.
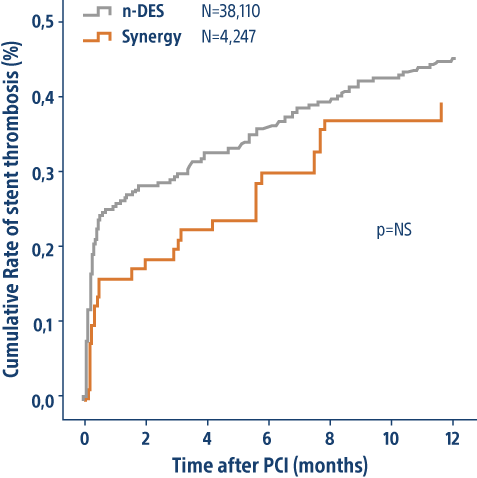
SYNERGY BP Stent showed numerically lowest ST rates compared to other DES despite being used in patients with more complex disease
| SYNERGY Stent | DES | p | |
|---|---|---|---|
| N patients | 4,247 | 38,110 | <0.01 |
| Class B2/C Lesions | 59% | 54% | <0.01 |
| LM | 6.9% | 6.1% | NS |
| CTO | 4.8% | 4.1% | NS |
| 2VD | 30.0% | 29.4% | NS |
Diabetes | 23.1% | 21.9% | <0.01 |
Prior PCI | 31.2% | 29.2% | <0.01 |
3VD | 17.4% | 16.6% | <0.01 |
Stent Diameter | 2.95 mm | 3.00 mm | <0.01 |
Stent Length | 22.32 mm | 20.32 mm | <0.01 |