SYNERGY™ & SYNERGY MEGATRON™
EES PtCr Coronary Stent System
EVOLVE
291 patients.
PROMUS Element vs. SYNERGY vs. SYNERGY Half-Dose (1:1:1).
Primary Endpoint: 6 month Late Loss + Composite Safety @ 30 days
EVOLVE II RCT
1684 patients, 125 sites, 16 countries.
PROMUS Element Plus vs. SYNERGY (1:1) single-blind trial.
Primary Endpoint: 12 month TLF
EVOLVE II QCA
100 Patient Registry, 12 sites (Australia, Japan, New Zealand, Singapore).
Primary Endpoint: 9 month in-stent Late Loss
EVOLVE China
400 patients, 15 sites.
PROMUS Element Plus vs. SYNERGY (1:1)
Primary Endpoint: 9 month Late Loss
EVOLVE China
Prospective, ~2000 patients, ~120 global sites.
Co-primary Endpoints: (1) Death/MI and (2) ARC definite/probable ST
SYNERGY™ Stent Reported Low Event Rates in a Leading Complex PCI Center in Belfast, Ireland
| SYNERGY Stent | |
|---|---|
| N patients | 185 |
| Class C Lesions | 81.1% |
| Turned down for surgical revasc | 23.8% |
| LM PCI | 14.1% |
| Multi-vessel Disease | 33% |
CTO | 33% |
3 months DAPT | 78% |
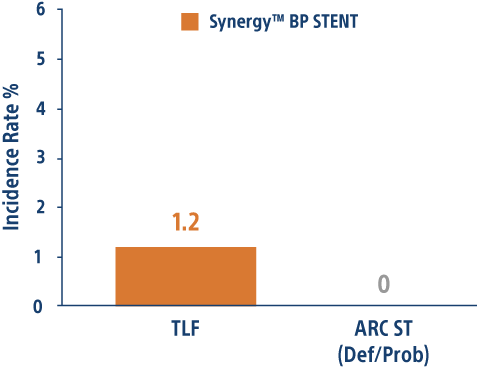
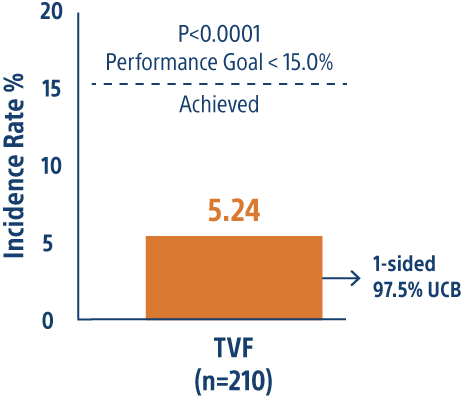
CONSISTENT CTO Trial 12-month Results
Trial Design:
Single-arm, 6 center study of 210 CTO patients evaluating primary end-point of TVF vs performance goal with the SYNERGY™ BP-DES.
Key Trial Details:1
- 90.5% of patients had pre-PCI IVUS
- 90% CTO success rate
- Very complex and symptomatic patients
- JCTO Score of 2.4
- Mean stent length 85.6 mm
- 21% diabetic patients
- Significant QoL gains at 12-months following successful CTO PCI
Presenter conclusion:
SYNERGY BP-DES highly effective in CTO PCI