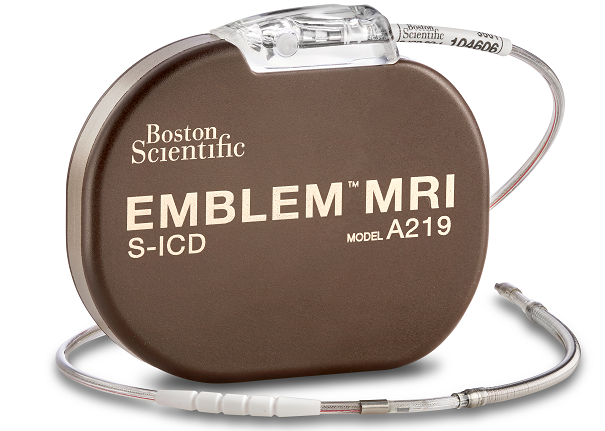
EMBLEM™ MRI S-ICD System
Subcutaneous Implantable Defibrillator
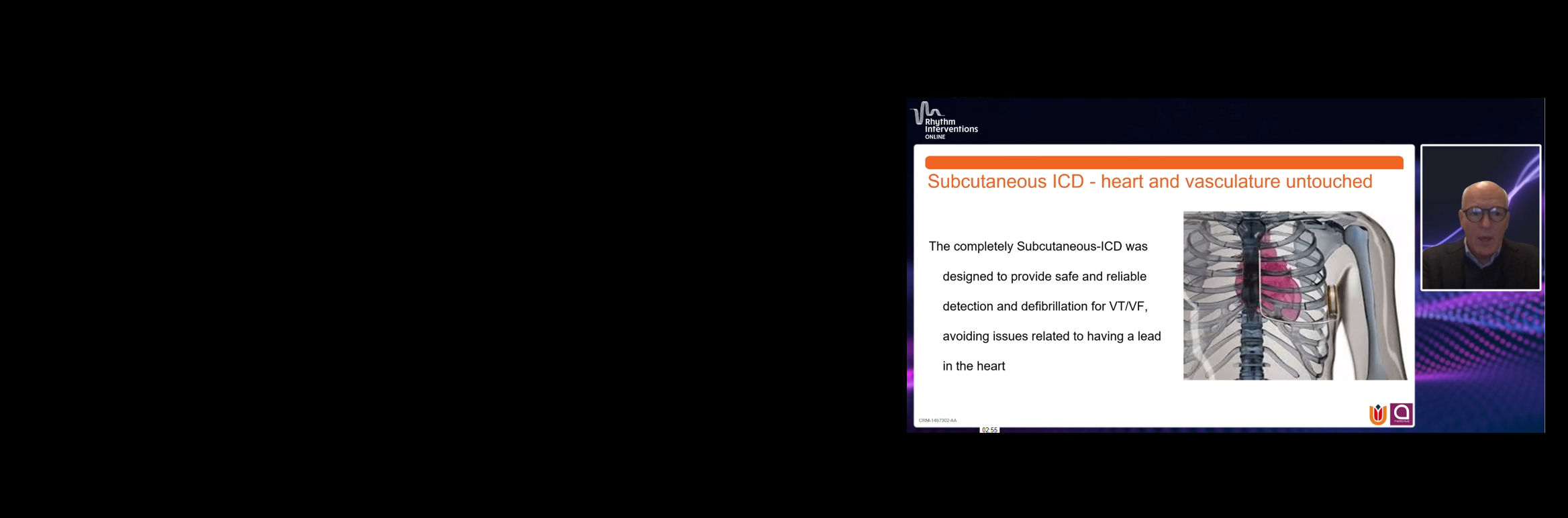
The only extrathoracic device of its kind
The S-ICD system was designed to address the main complications that may occur in Transvenous ICDs: Lead Complications and Infection.1
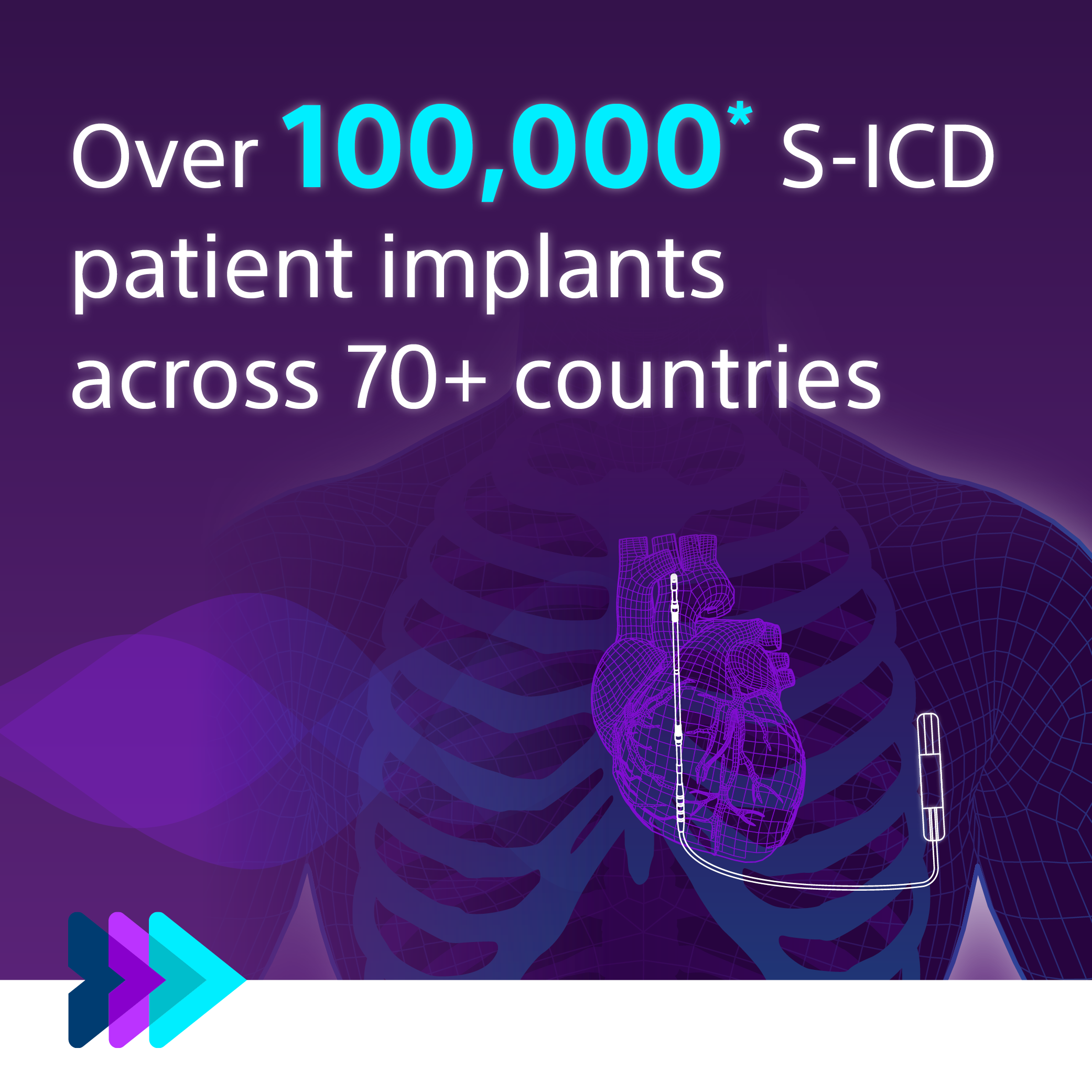
More than 18 years of clinical data shows that the S-ICD is safe and effective and has comparable performance to TV-ICD in treating VT/VF.2,3
The S-ICD System leaves the heart and vasculature UNTOUCHED, avoiding the complications associated with transvenous leads.
Product Details
The EMBLEM MRI S-ICD protects patients from sudden cardiac death while avoiding the serious risks of more-invasive ICD options.4-10
More than 20 years of clinical data show us that S-ICD:
- is safe and effective
- is superior to TV-ICD showing lower and less severe lead-related complications
- has a very low rate of inappropriate therapy
- is suitable for all patients without the need for pacing

S-ICD is Recommended in both US and EU Guidelines
| Guidance | 2017 AHA/ACC/HRS Guidelines4 | 2015 ESC Guidelines | For S-ICD patients... |
|---|---|---|---|
| Class I | With high risk of infection, including Diabetic patients (up to 35% of the ICS population)5 | ||
| Class IIa | Without need of pacing (CRT, bradycardia, ATP) |

ATLAS Result: S-ICD is superior to TV-ICD 10

The EMBLEM™ S-ICD has proven superiority to TV-ICD in preventing the serious complications associated with invasive leads as early as 6 months after implant, as shown in the ATLAS trial.