Clinical overview: SYNERGYTM Bioabsorbable Polymer Stent Platform
The SYNERGYTM Bioabsorbable Polymer (BP) Stent Platform has been studied in over 35,000 patients across various patient and lesion complexities.
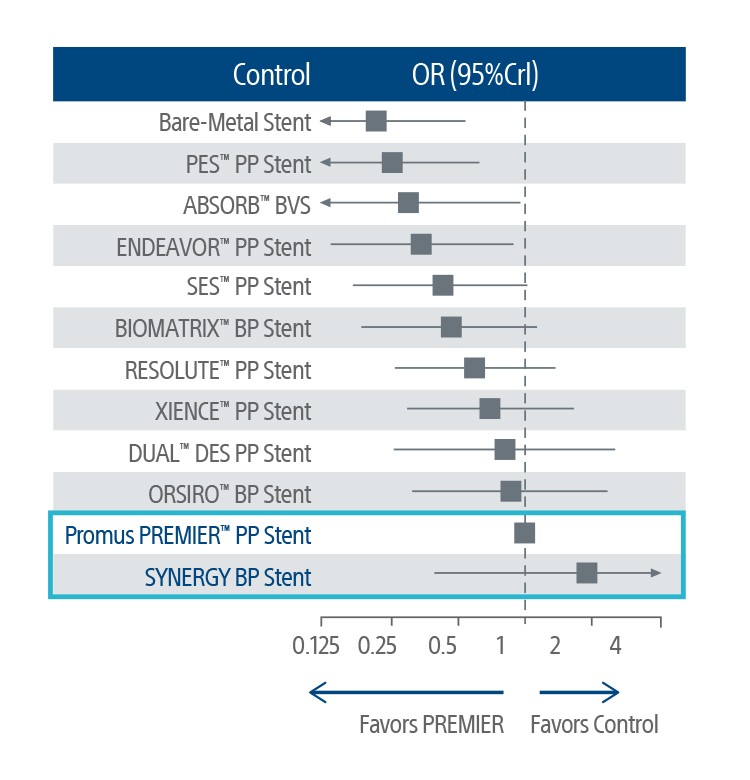
Heal With Confidence: Leading in Complex Patients
The SYNTAX II Trial utilizing the SYNERGY BP Stent, physiological assessment, IVUS guidance, and contemporary state-of-the art PCI techniques demonstrated CABG-like outcomes in patients with three-vessel disease at 5 years.*†¹
Low rates of revascularization, peri-procedural MI, and acute ST suggests that contemporary technologies might help in reducing procedural related complications.
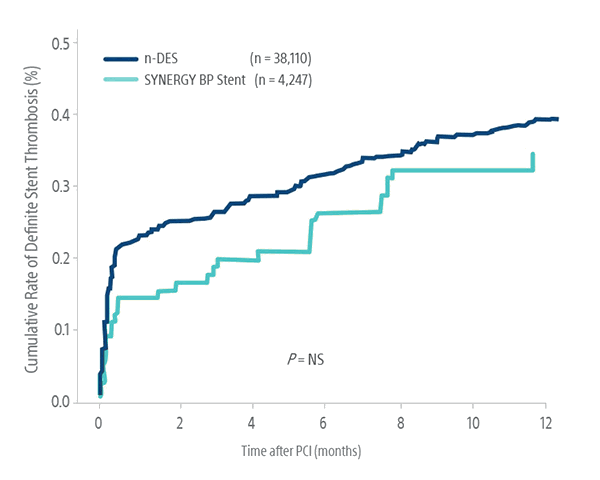
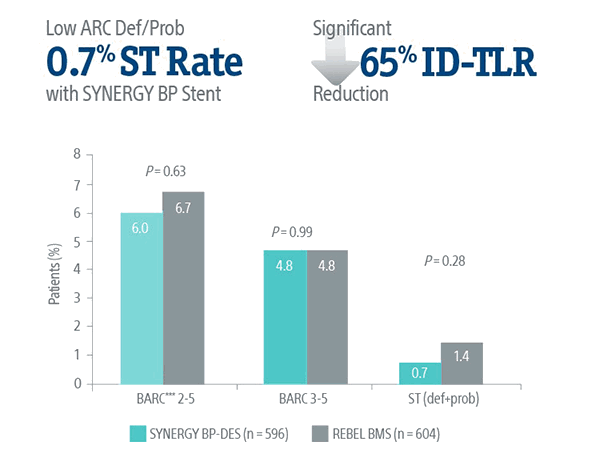
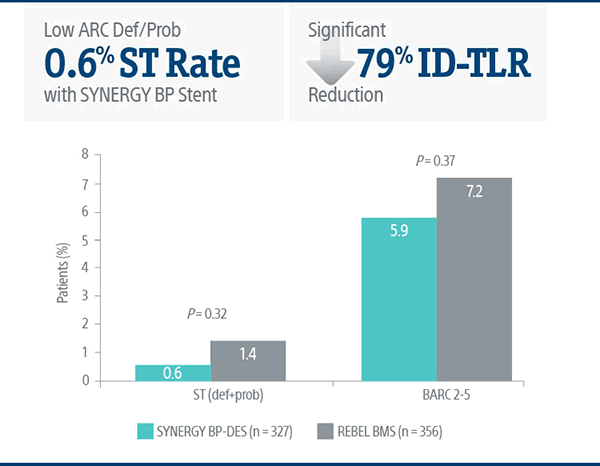
Consistently Low ST Rates
Leading On Studying Short DAPT
Supporting well-constructed prospective Short DAPT clinical trials with over 5,000 patients to study the SYNERGY BP Stent in various complex patient populations.
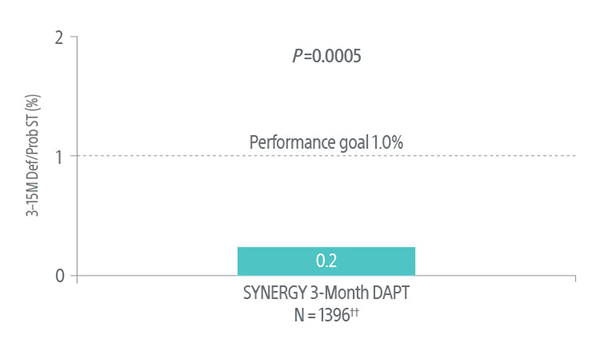
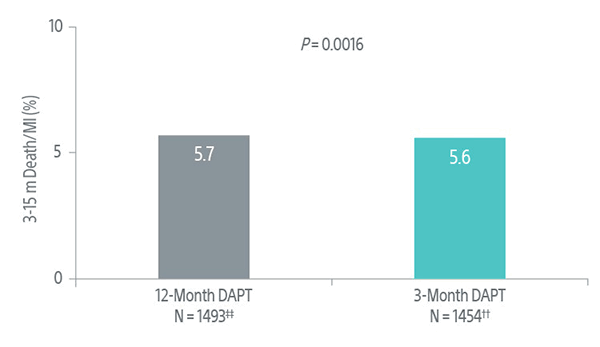
EVOLVE Short DAPT Trial: Primary Endpoint Results 12 Months Post-DAPT Discontinuation
Studied the safety of discontinuing DAPT at 3-months in high bleeding risk (HBR) patients using the SYNERGY BP Stent.4
SENIOR Trial: Safety Data at 2-Years
The SYNERGY BP Stent continued to show superior results versus REBEL BMS with short BMS-like DAPT regimen at 2-years.5
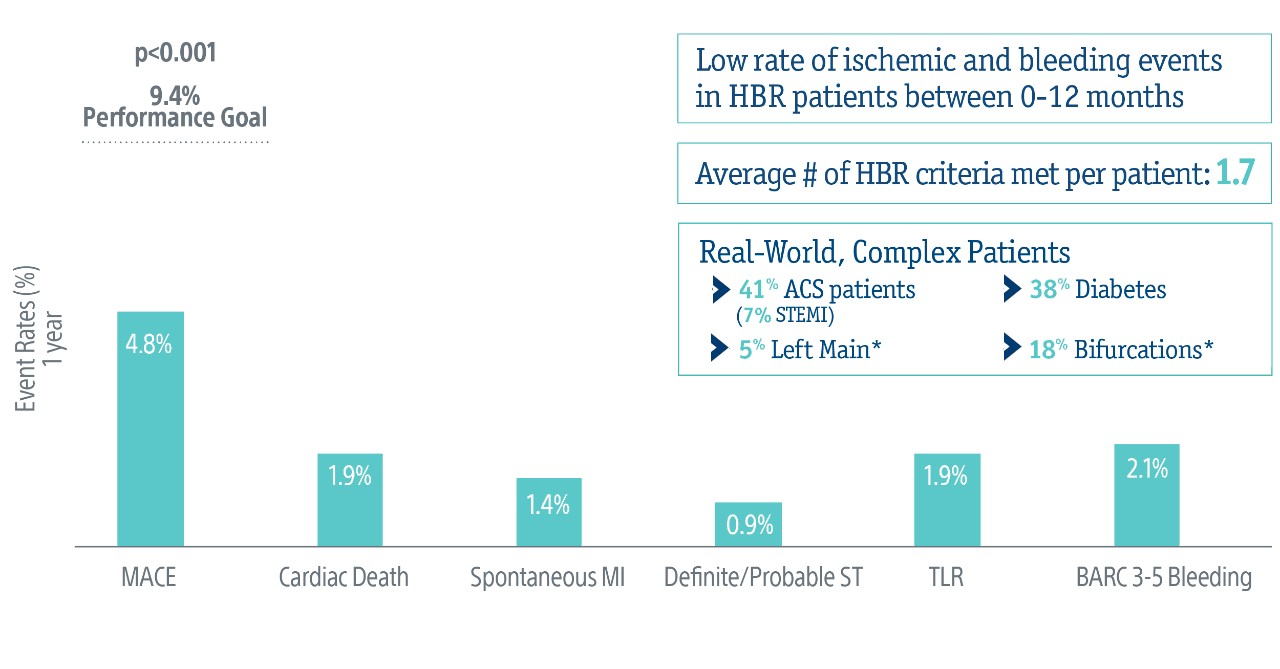
POEM Trial: 1-year Results
At 1 year, in an all-comers HBR patient population, the SYNERGY BP Stent with 1-month DAPT beat the performance goal derived from LEADERS FREE6:
Excellent Outcomes in Long Lesions
EVOLVE 48 Trial: Final 2-year results
Supports the safety and effectiveness of the SYNERGY XD 48 mm BP Stent for the treatment of long lesions.†††7:
- 100 patients
- 15 sites across US, Europe, and New Zealand
- 100% B2/C lesions
- 27% diabetes mellitus
Excellent Outcomes in STEMI Patients
CLEAR SYNERGY Stent Registry: Primary Endpoint Results
At 1 year, in an international, multicenter registry, the SYNERGY BP Stent demonstrated low event rates in this high-risk STEMI patient population.8
Education & Training with EDUCARE