Proven Performance
Low need for Brady/ATP
S-ICD is recommended for most ICD-indicated patients, without the need for pacing, in the following guidelines:
- Class I, 2017 AHA/ACC/HRS Guidelines2
- Class IIa, 2017 AHA/ACC/HRS and 2015 ESC Guidelines2,3
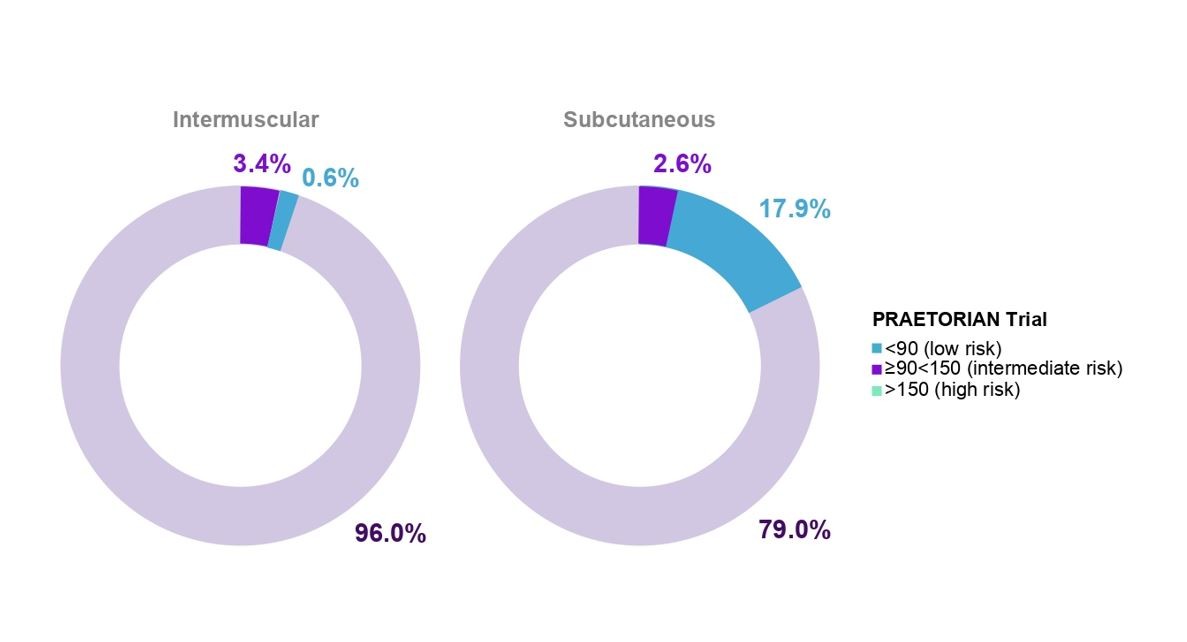
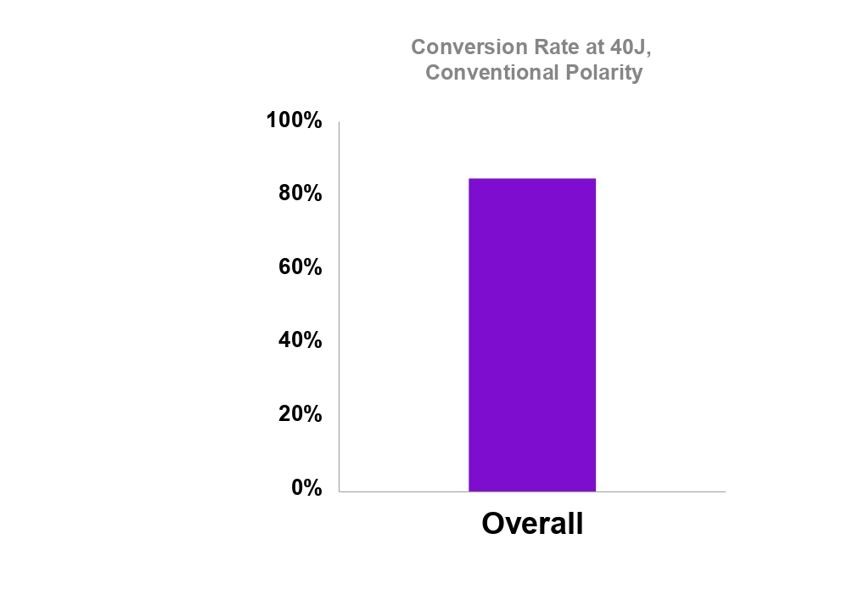
Although S-ICD lacks Brady pacing and ATP functionality, there is relatively low demand for these in patients with an ICD indication receiving an S-ICD6,8,10-12
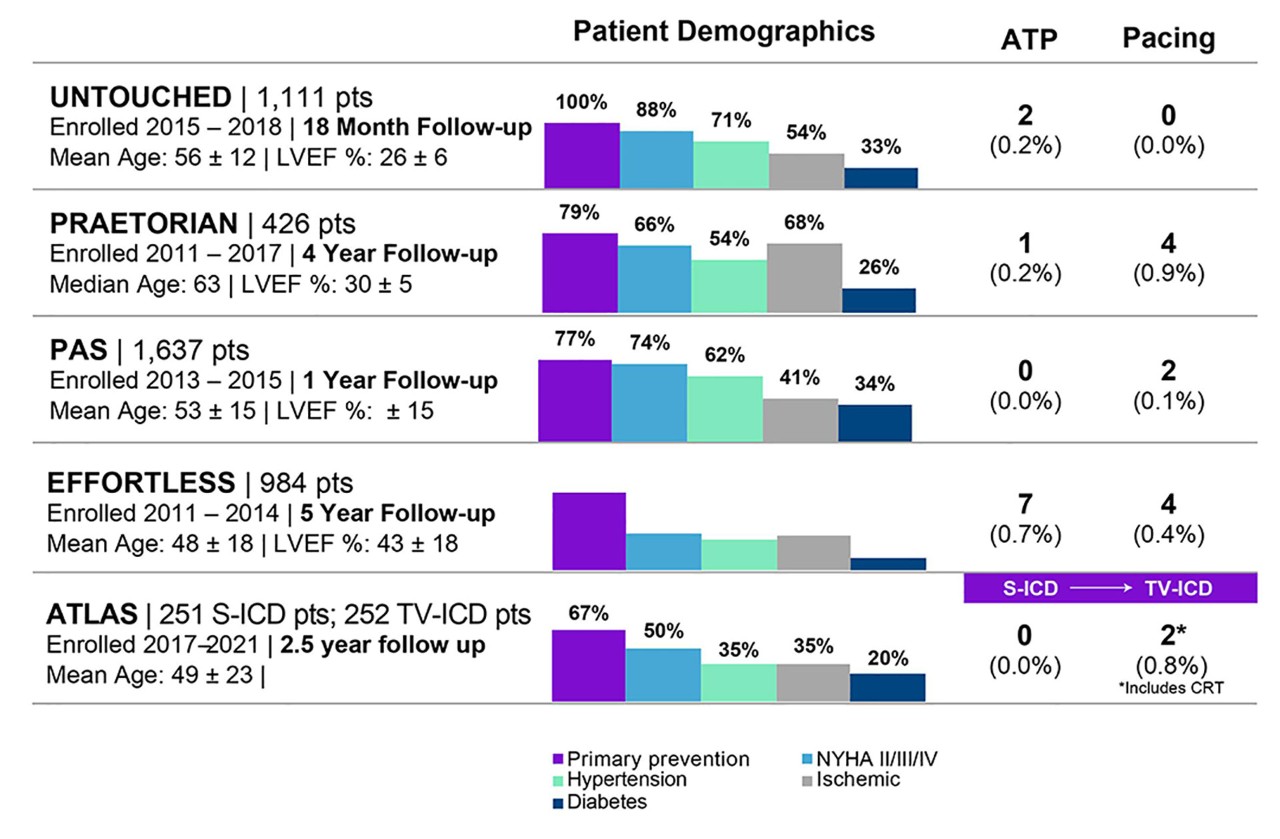
The MODULAR ATP study, which is currently enrolling patients, is designed to demonstrate the safety, performance and effectiveness of the mCRM™ System (EMBLEM™ S-ICD System and EMPOWER™ Modular Pacing System) for those few patients who may need Brady pacing or ATP at implant, or may develop a need in the future.
All EMBLEM™ S-ICDs are, by design, compatible with the EMPOWER™ Leadless Pacemaker*.

Real-World Data
Implantation technique
Optimised programming
Long-term use
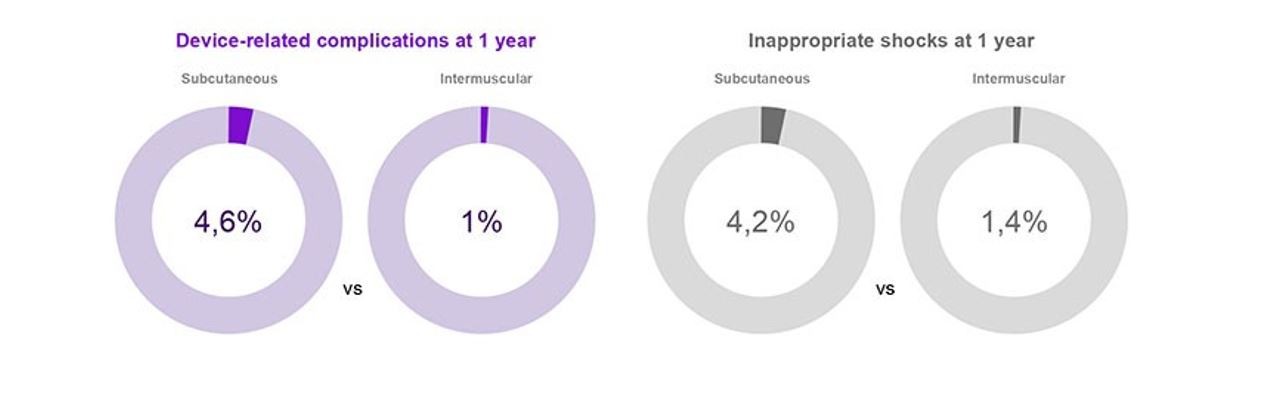
The HONEST independent cohort study, supported long-term S-ICD use in a real-world setting, with 4,924 patients enrolled across 150 centres in France.12
Similar to the PRAETORIAN Trial, S-ICDs enrolled in the HONEST cohort demonstrated high efficacy, low rates of complications, as well as low inappropriate shock rates; in long-term real-world settings, in a varied patient cohort.