Innovate, single-use design enhances safety
Despite adherence to rigorous disinfection and reprocessing protocols, multiple infection outbreaks worldwide have been linked to contaminated duodenoscopes used in endoscopic retrograde cholangiopancreatography (ERCP) procedures. As a result, the FDA has called for duodenoscopes with innovative designs to enhance safety, including scopes with disposable components or fully disposable duodenoscopes.1
The EXALTTM Model D Single-Use Duodenoscope completely eliminates the concern for device cross-contamination and the risk of patient-to-patient infection due to ineffective reprocessing.*
*As defined by the American Society for Gastrointestinal Endoscopy.
Complex cleaning processes increase post-ERCP infection risk
From pre-cleaning to drying, there can be more than 100 distinct steps to reprocessing a reusable duodenoscope—all of which introduce the opportunity for patient cross-contamination. Only a disposable duodenoscope like EXALT Model D eliminates the risks of cross-contamination associated with ineffective reprocessing.
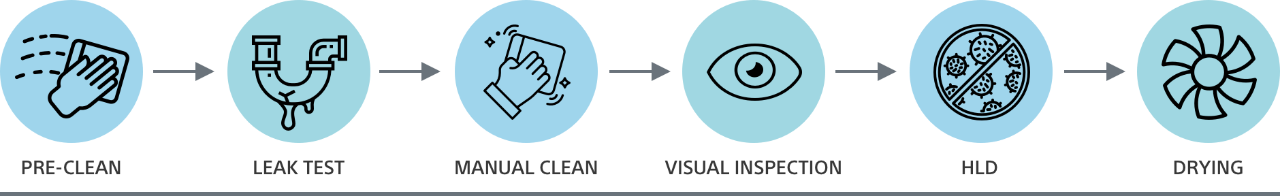
Single-use duodenoscopes vs. single-use endcaps
Clinical endoscopy technologies such as single-use endcaps and single-use duodenoscopes are designed to help reduce the risk of cross-contamination due to ineffective reprocessing. Yet, while disposable components like endcaps may lower the risks of infection, only single-use duodenoscopes – like EXALT Model D – completely eliminate post-ERCP infection risks as a result of cross-contamination from ineffective reprocessing.
| Benefit* | Exalt Model D | Single-Use Endcaps |
| Eliminates risk of patient infection due to ineffective reprocessing | Yes | No |
| Eliminates duodenoscope reprocessing training and compliance | Yes | No |
| Decreases waste from reprocessing such as disinfecting consumables | Yes | No |
| Enhances infection prevention efforts aligning with 2022 FDA Safety Communication** | Yes | Yes |
*As compared to reusable duodenoscopes. Assumes full conversion of all ERCP procedures using reusable duodenoscopes to instead using the EXALT Model D Duodenoscope.
** FDA News Release

Find out how EXALT Model D may help reduce infection risk due to ineffective reprocessing and benefit your facility
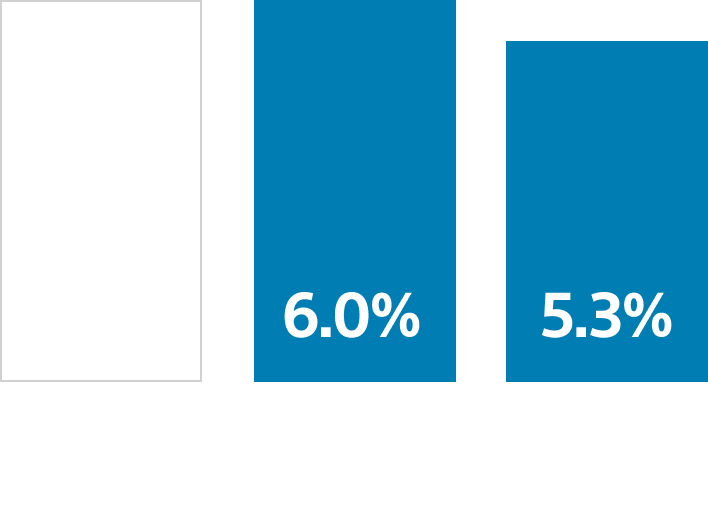
Meta-analysis of reusable duodenoscope contamination 2,3,4
A meta-analysis of 15 studies found that neither double high-level disinfection (HLD) nor ethylene oxide (EtO) gas sterilization eliminated the risk of contamination in reusable duodenoscopes that were considered patient ready.
![]()
15 Studies
![]()
13,112 Patient-ready duodenoscope
![]()
15.3% Contamination rate
Duodenoscope safety communications
The environment, equipment and guidelines that inform cleaning procedures are constantly changing, putting duodenoscope reprocessing into a state of transition.
In Europe, ESGE and ESGENA collaborated in 2019 to produce Position Statement on this topic, stressing that regardless of duodenoscope design, there are two crucial points:1,3
- Standardized and validated duodenoscope reprocessing should be performed by appropriately trained, dedicated, and competent staff;
- Microbiological surveillance and regular maintenance of duodenoscopes should be performed to identify any problems at an early stage.
In the United States, FDA recommended in 2019 to transition to duodenoscopes with innovative designs that range from disposable endcaps to fully disposable duodenoscopes.1
FDA Mandated surveillance studies 5,6
Due to a growing concern over the post-ERCP infection risks with reusable scopes, the FDA mandated post-market surveillance studies to monitor the effectiveness of duodenoscope reprocessing.

High-concern
|

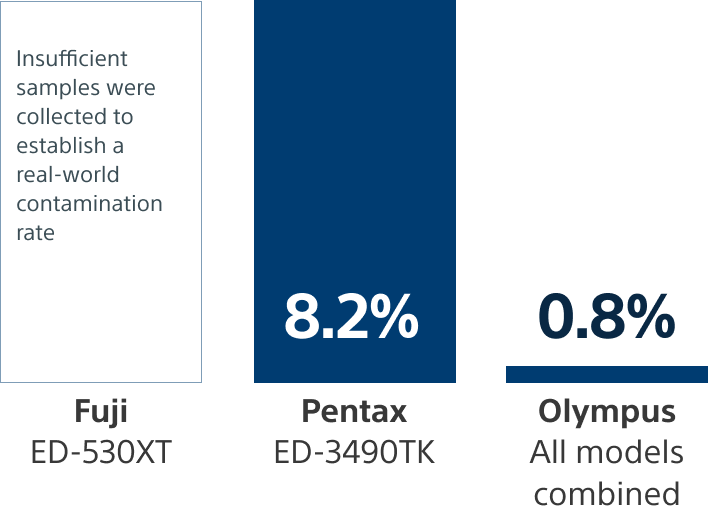
Low-concern
|
**Pentax: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm?t_id=355&c_id=3727
***Olympus 522 site: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm?t_id=354&c_id=3726
EXALT™ Model D
Single-Use Duodenoscope
delivers robust stability, maneuverability
and control in ERCP procedures.
![]()
Stay up to date
Sign up to receive periodic emails about EXALT Model D case studies, clinical data and more.
![]()
Connect with a rep
Request a rep to learn how EXALT Model D may help you address infection risks and improve patients’ lives.

















