Setting up your Communicator with a USB cellular adapter
It’s important to set up your Communicator right away so it can send information collected from your implanted device to your health care team.
If your Communicator is already setup, see 4G USB Cellular Setup Guide.
Set-up instructions
- Place the monitor in a location where there is a good cell signal
- The USB cellular adapter does not use your personal cell phone
1. Attach the power cord
Insert the power cord (included) into the jack as shown below and plug the cord into an electrical outlet that is easily accessible.
- The LATITUDE™ indicator will flash yellow for up to 1 minute
- All the Communicator indicators will light for approximately 1 second
- If the LATITUDE indicator is not lit, check that both ends of the power cord are plugged in firmly and the light on the power cord is lit

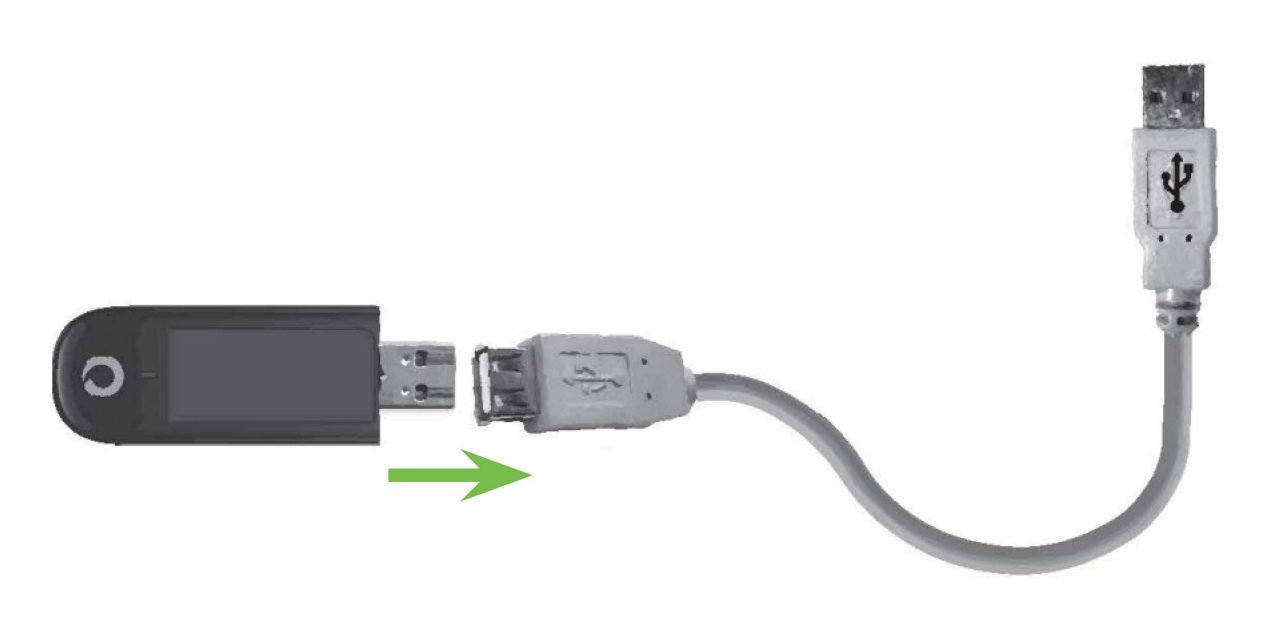
2. Insert the metal end of the USB cellular adapter into the female end of the USB extension cable
Note: There will be a slight gap between the adapter and the cable.
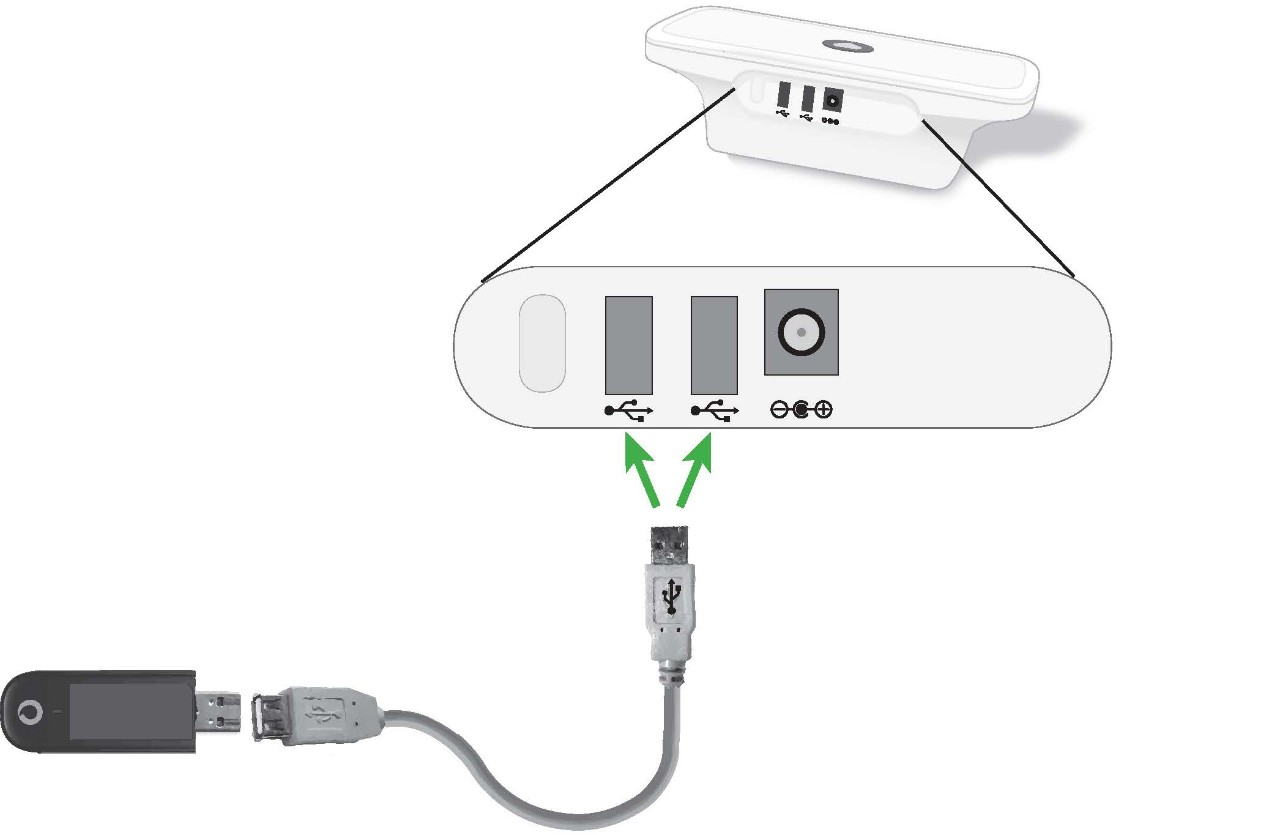
3. Connect the USB extension cable into either one of the USB ports on the back of your Communicator
4. Press the flashing Heart button to start the setup process

5. Watch your Communicator’s wave lights
Your Communicator’s wave lights will flash green in sequence and repeat for several minutes.
Collecting data from your implanted device

Sending data

If the LATITUDE Indicator flashes yellow, a software update may be occurring. Wait several minutes for the Heart button to flash, then press it again. This may happen multiple times. Press the Heart button each time it flashes.

6. Success
Troubleshooting
Refer to the LATITUDE NXT Communicator Quick Reference Guide or the troubleshooting sections of the LATITUDE Communicator Patient Manual or download the free MyLATITUDE Patient App.