If you are not implanted with a pacemaker device, please visit the pacemaker procedure page for more information.
Boston Scientific accounts are for healthcare professionals only.
Boston Scientific accounts are for healthcare professionals only.
Create an account to access online training and education on EDUCARE, manage your customer profile, and connect with customer support and service teams.
My Boston Scientific account
Access your online applications and manage your customer profile.
Quick Links
Call customer care
If you are not implanted with a pacemaker device, please visit the pacemaker procedure page for more information.
Your permanent Medical Device Identification (ID) Card will be mailed to you a few weeks after your implant. This wallet-sized card helps identify you as a patient with an implanted Boston Scientific medical device (pacemaker, defibrillator, or lead wire). If you do not receive your permanent card within eight weeks, call 1-866-484-3268 to order one.
If your name or address changes, or if you get a new heart doctor, let us know so we can update our records.
You can download and print information about your pacemaker to share with your family by selecting your Boston Scientific pacemaker model on the pacemaker resources page. These info sheets include answers to commonly asked questions about pacemakers and a summary about your device—including a photograph and dimensions. You may also want to share your patient manual with them. If you would like to request a new manual, call Boston Scientific Patient Services at (866) 484-3268.
Your doctor can learn about changes to your heart condition from your activity level, heart events, and treatments from the information stored in your device.
Since your device is on 24 hours a day, it can provide a good picture of your heart rates and rhythms, your activity level, and how much help your heart needs to beat normally. If you receive therapy from your device, your doctor can see pictures (electrograms) about what happened.
Just in case you have an arrhythmia, your pacemaker keeps the last 10 seconds of your heartbeats in memory for each of the leads in your heart. This is called the Onset segment of an arrhythmia. When you have an arrhythmia, your doctor can see what happened just before it occurred. This helps your doctor evaluate your heart rhythm, adjust your medications, or make changes to your device settings, if needed.
Download details about your specific pacemaker, including its size, maintenance and how long you can expect it to last.
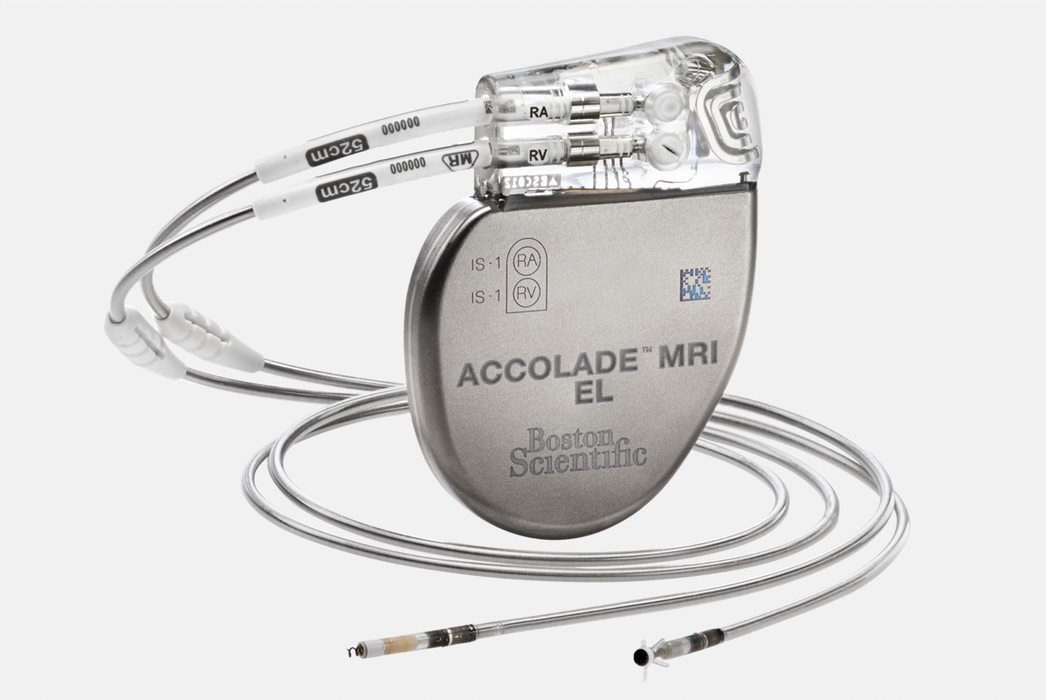
Dual-chamber pacemakers with advanced cardiac pacing and remote monitoring.

The ADVANTIO pacemaker defines a new era in pacing. This pacemaker leverages key aspects of our innovative high-voltage platform and incorporates new features, therapies and diagnostic options.
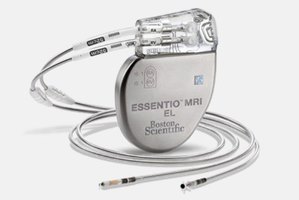
ESSENTIO MRI models can be used as part of the ImageReady™ MR-Conditional Pacing System for safe and effective scanning in 1.5T and 3T MRI environments when MRI Conditions of Use are met.
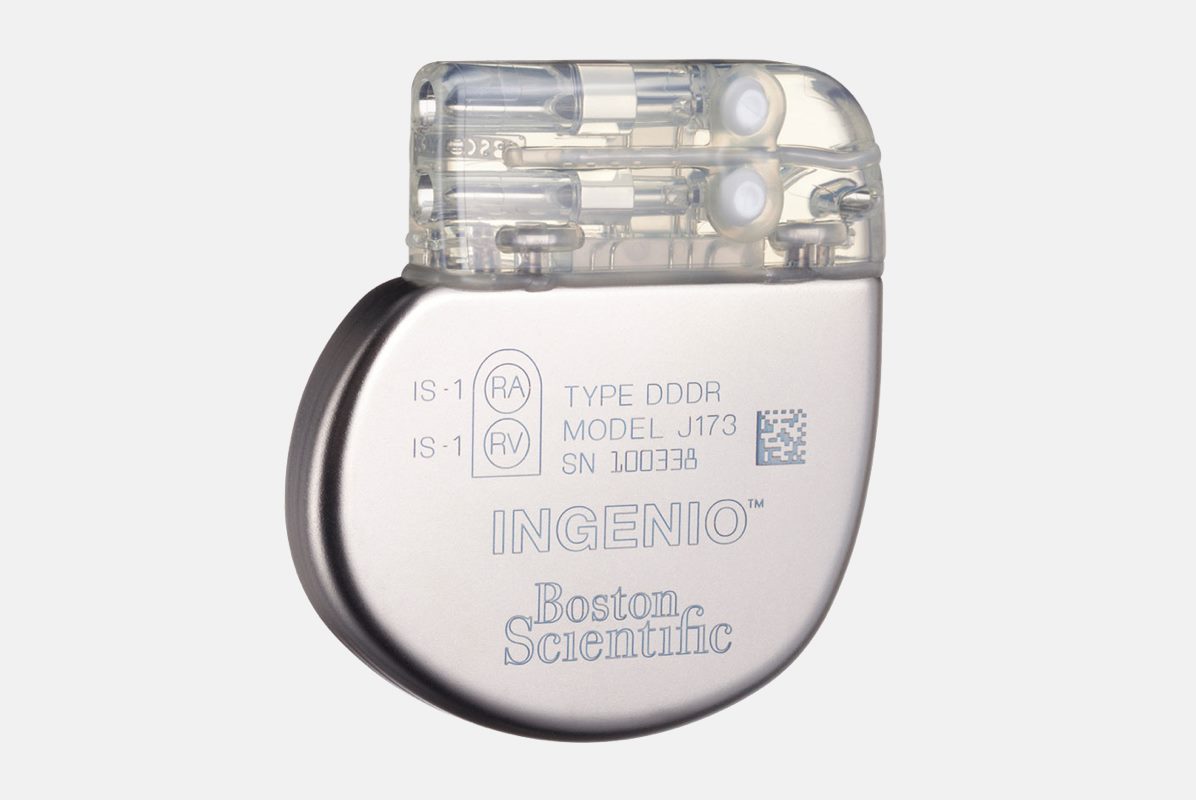
The INGENIO pacemaker defines a new era in pacing. This pacemaker leverages key aspects of our innovative high-voltage platform and incorporates new features, therapies and diagnostic options.
Our patient services team is here to support you throughout your journey.
Pacemakers
Important Safety Information
A pacemaker system is designed to monitor and treat your heart rhythm problems, greatly reducing the risks associated with them. These devices are sensitive to strong electromagnetic interference (EMI) and can be affected by certain sources of electric or magnetic fields. Some of the risks encountered during the implant procedure include, but are not limited to, the following: Bleeding, formation of a blood clot, damage to adjacent structures (tendons, muscles, nerves), puncture of a lung or vein, damage to the heart (perforation or tissue damage), dangerous arrhythmias, heart attack, stroke, death. Some of the risks encountered after the system is implanted may include, but are not limited to, the following: Infection, erosion of the skin near your device, lead(s) may move out of place in the heart, device may move from the original implant site, difficulty coping with having an implanted device. The device might be prevented from pacing due to electromagnetic interference. Electrodes on the lead or the pacing pulses may cause an irritation or damaging effect on the surrounding tissues, including heart tissue and nerves. You may receive pacing therapy when it is not needed (unnecessary therapy). The device might not be able to detect or appropriately treat your heart rhythms. The device may exhibit malfunctions that may result in lost or compromised ability to deliver therapy. You may experience some discomfort from the incision as you recover from the surgery. You may experience some discomfort from the incision as you recover from the surgery. With all medical procedures there are risks associated. In rare cases device failure or death can occur. Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of this system. To obtain a copy of the device Patient Handbook for more detailed device safety information, go to www.bostonscientific.com, or you can request a copy by calling 1-866-484-3268 or writing to Boston Scientific, 4100 Hamline Ave. N., St. Paul, MN 55112. Rx only
Device Quality and Reliability
It is Boston Scientific’s intent to provide implantable devices of high quality and reliability. However, these devices may exhibit malfunctions that may result in lost or compromised ability to deliver therapy. Refer to Boston Scientific’s CRM product performance report on www.bostonscientific.com for more information about device performance, including the types and rates of malfunctions that these devices have experienced historically. While historical data may not be predictive of future device performance, such data can provide important context for understanding the overall reliability of these types of products. Also, it is important that you talk with your doctor about the risks and benefits associated with the implantation of a device.
92481216 B