AGENT™
Drug-Coated Balloon
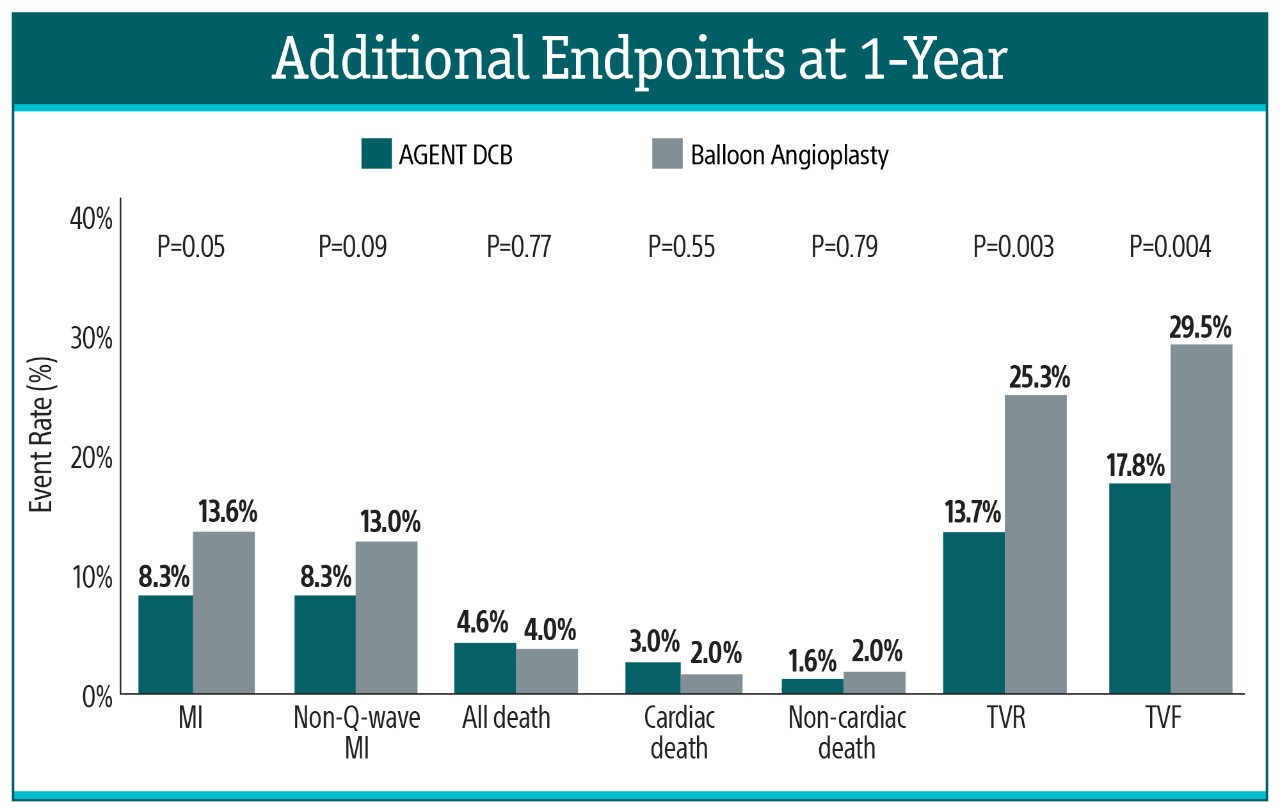
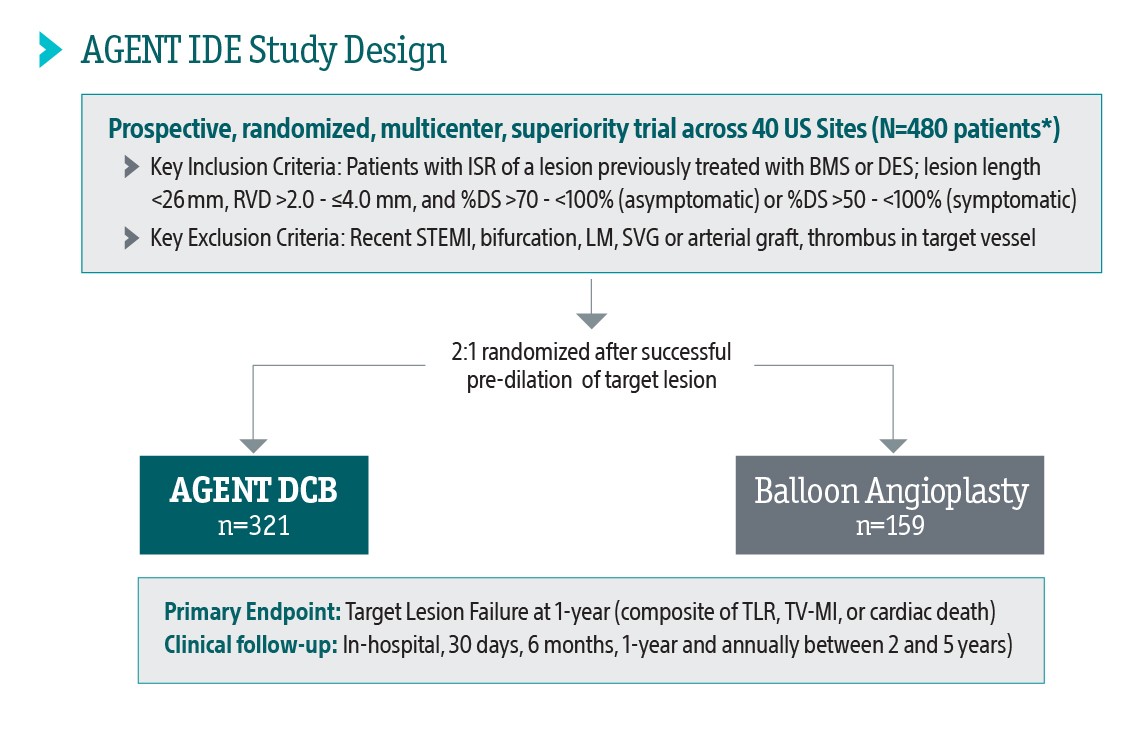
The AGENT IDE Clinical Trial showed that AGENT DCB is superior to conventional balloon angioplasty in reducing target lesion failure for the treatment of coronary in-stent restenosis (ISR).1
AGENT IDE Clinical Trial
Primary Endpoint²
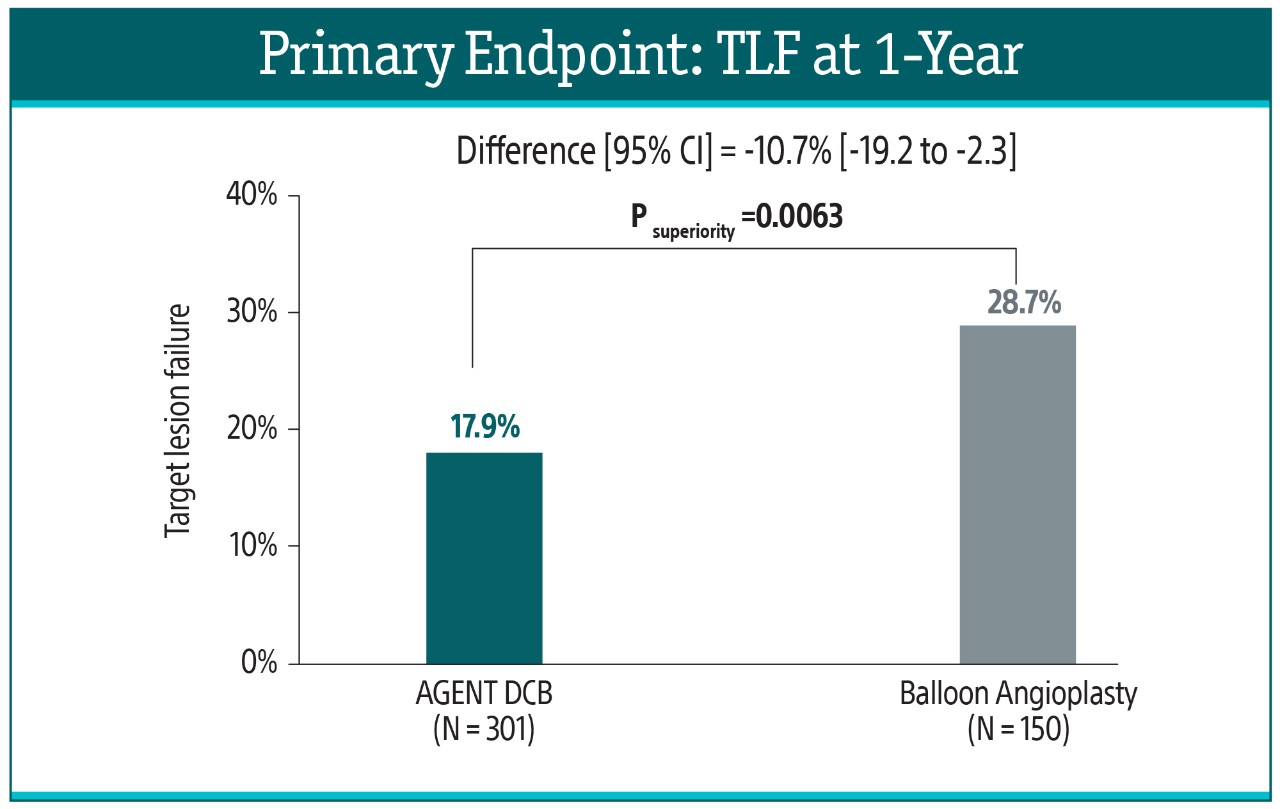

Additional Endpoints²
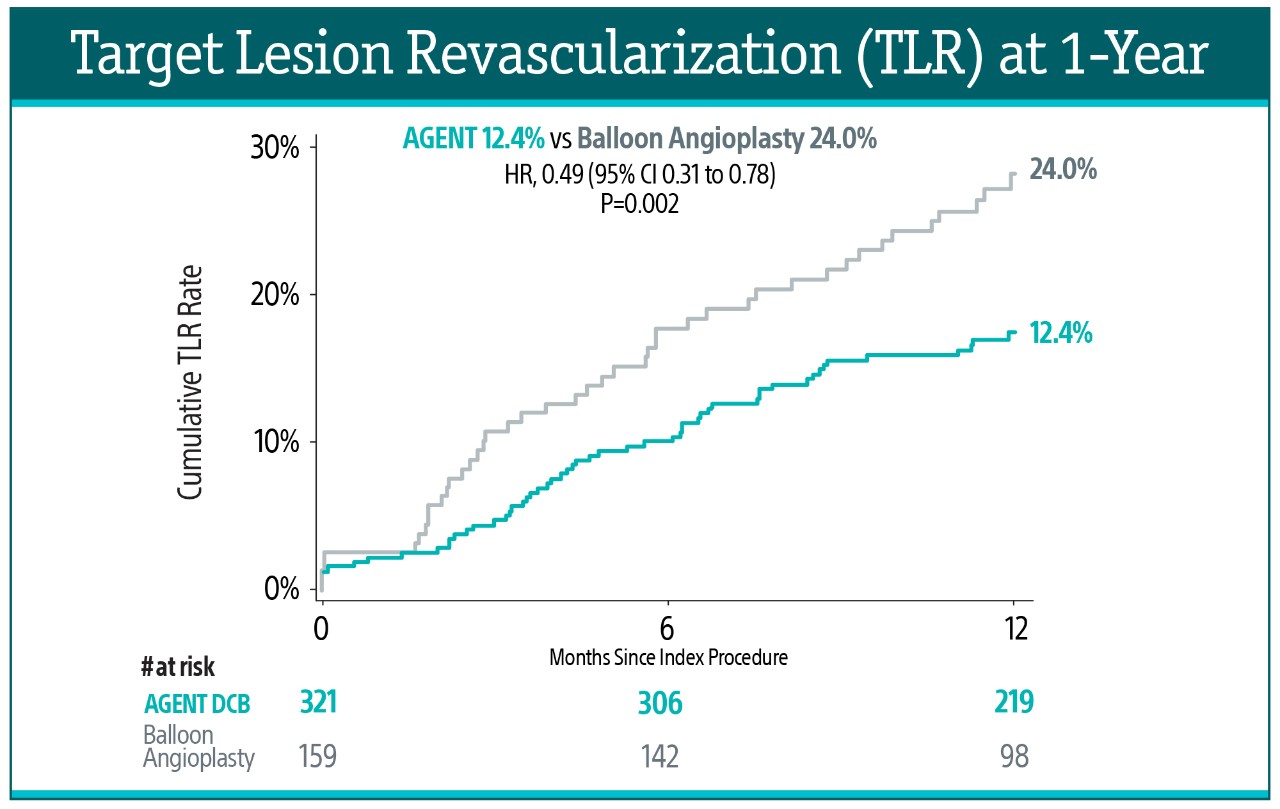
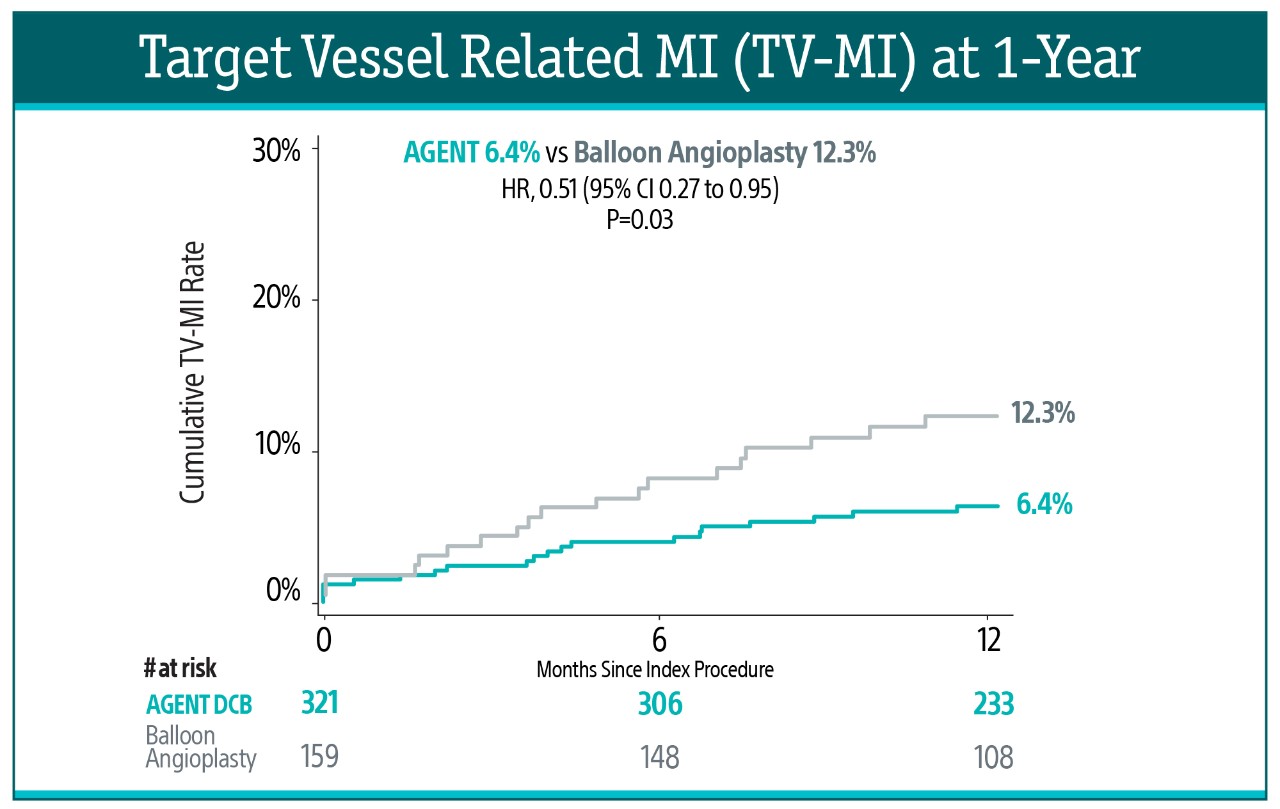
At 1-year, AGENT DCB also demonstrated statistically lower event rates:
- 51% risk reduction in TLR (12.4% vs. 24.0%, P=0.002)
- 49% risk reduction in TV-MI (6.4% vs 12.3%, P=0.03)
- Zero definite/probable ST (0.0% vs. 3.9%, P=0.001)


















-v4.jpg)