MOTION multicentre study overview
Cryoablation for palliation of painful bone metastases
Radiology: Imaging Cancer 2021; 3(2):e200101 Jack W. Jennings, MD, PhD • J. David Prologo, MD • Julien Garnon, MD • Afshin Gangi, MD, PhD • Xavier Buy, MD • Jean Palussière, MD • A. Nicholas Kurup, MD • Matthew Callstrom, MD, PhD • Scott Genshaft, MD • Fereidoun Abtin, MD • Ambrose J. Huang, MD • Jason Iannuccilli, MD • Frank Pilleul, MD, PhD • Charles Mastier, MD • Peter J. Littrup, MD • Thierry de Baère, MD • Frédéric Deschamps, MD.
This multicenter, prospective, single arm, phase II study examined the impact of the treatment of a painful bone metastatic lesion in each patient.
Study objective and design
- The primary objective was to evaluate the efficacy of cryoablation for pain palliation of bone metastases from baseline to 8 weeks after cryoablation in worst pain in the last 24 hours.
- Separate evaluations of ancillary efficacy endpoints were also made through 24 weeks.
Study design
- Multicentre, prospective, single arm, phase II study
- 11 centres: 4 in EU and 7 in US;
- Conducted from February 2016 to March 2018;
- N= 66 patients with painful bone metastases treated, 65 available for follow-up;
- Patient follow-up at 1, 4, 8, 12, 16, 20, and 24 weeks after the cryoablation procedure;
- Treatment of 1 painful bone metastatic lesion for each patient;
- Primary efficacy objective: change from baseline to 8 weeks after cryoablation in worst pain in the last 24 hours as measured by the BPI-SF scale;
- Complications were monitored for 30 days post procedure;
- Hospital stay: median of 26.6 hours (range 19.4 – 45.8 hours).
Patient and tumour characteristics
| Characteristics | Participants (n = 66) |
| Primary cancer diagnosis | |
| Lung cancer | 19 (28.8%) |
| Breast cancer | 9 (13.6%) |
| Other* | 13 (19.7%) |
| Kidney cancer | 8 (12.1%) |
| Colon cancer | 5 (7.6%) |
| Prostate cancer | 4 (6.1%) |
| Sarcoma | 3 (4.5%) |
| Thyroid cancer | 3 (4.5%) |
| Stomach cancer | 2 (3.0%) |
| Prior cancer treatments | |
| No prior cancer treatment | 7 (10.6%) |
| Prior systemic chemotherapy | 50 (75.8%) |
| Prior radiation for bone metastases (index tumour) | 28 (42.4%) |
| Prior hormonal treatment (not restricted to bone metastases) | 12 (18.2%) |
| Prior targeted molecular therapy for bone metastases | 9 (13.6%) |
| Prior ablation therapy for non-index bone tumour(s) | 6 (9.1%) |
| Prior bisphosphonate treatment for bone metastases | 5 (7.6%) |
| Index tumour location | |
| Rib | 16 (24.2%) |
| Illium | 13 (19.7%) |
| Pelvis | 8 (12.1%) |
| Other | 6 (9.1%) |
| Chest wall (rib with non-rib soft tissue) | 4 (6.1%) |
| Acetabulum | 3 (4.5%) |
| Sacrum | 3 (4.5%) |
| Scapula | 3 (4.5%) |
| Ischium | 3 (4.5%) |
| Sternum | 3 (4.5%) |
| Humerus | 2 (3.0%) |
| Femur | 1 (1.5%) |
| Vertebra | 1 (1.5%) |
| Index tumour composition | |
| Predominantly lytic (osteolytic) disease | 48 (72.7%) |
| Mixed | 11 (16.7%) |
| Predominantly sclerotic (osteoblastic) disease | 6. (9.1%) |
Method
Study sites used a standard cryoablation protocol including two freeze-thaw cycles.
If the operator felt that another cycle would improve coverage and local control, it was performed in select cases.
Participants were not denied needed therapy for pain; however, those who received additional targeted therapies to the index tumour were excluded. Pain improvement was evaluated using a single item from the BPI-SF questionnaire completed by participants which asked participants to evaluate the level of the “worst pain in the last 24 hours.”
The primary effectiveness endpoint was the change from pre-treatment baseline rating of worst pain in the last 24 hours to post treatment week 8 rating. A clinically meaningful change for this item was defined as a reduction of at least 2 points.
Results
Sixty-six patients were included in the intention-to-treat (ITT) population in which cryoablation was attempted.
Baseline patient and tumour characteristics are shown in Table 1 with a mean patient age of 60.8 years and predominant primary cancers of lung (28/8%), breast (13.6%), and kidney (12.1%) cancer of targeted bone metastases.
The majority of patients had received previous systemic therapies (75.8%) with 42.4% of patients previously receiving radiation therapy, and only 10.6% of patients with no prior cancer treatments.
Change in worst pain in last 24 hours in 24 weeks
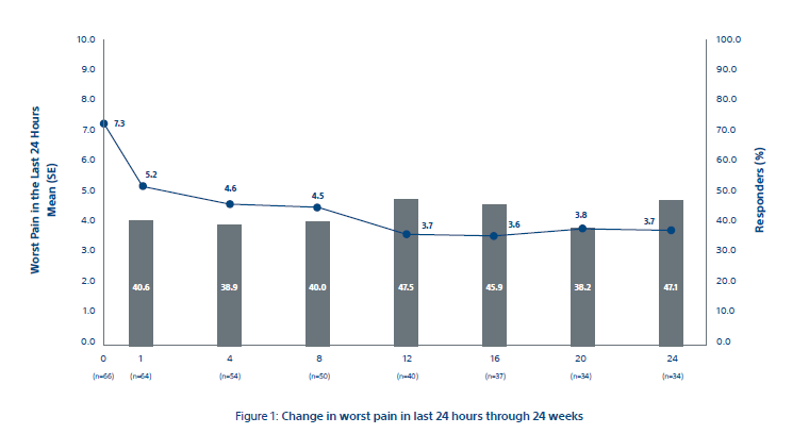
Figure 1
*The primary efficacy endpoint of mean change in worst pain in last 24 hours from baseline to week 8 was -2.61 ± 0.43 points (93% CI: - 3.45, -1.78) as shown in Figure 1. Clinically meaningful changes from baseline were observed at all time points after week 8.
Change in quality of life through 24 weeks
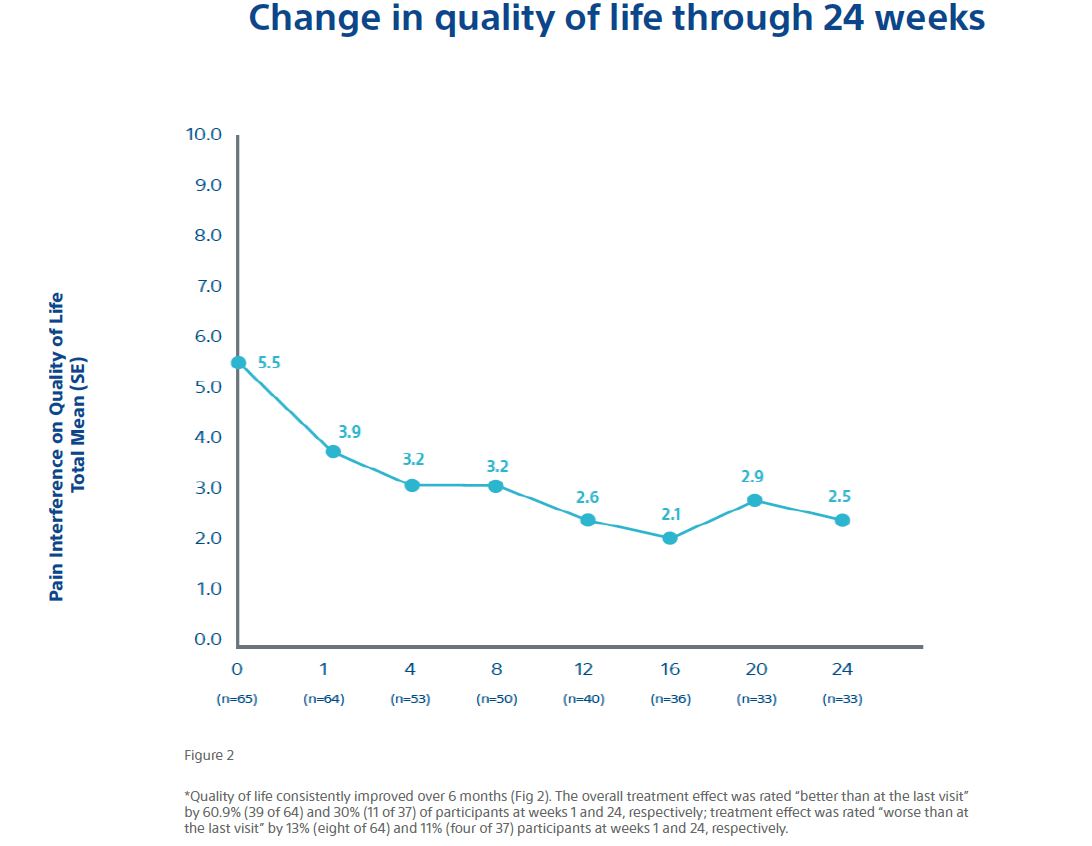
Figure 2
*Quality of life consistently improved over 6 months (Fig. 2). The overall treatment effect was rated “better than at the last visit” by 60.9% (39 of 64) and 30% (11 of 37) of participants at weeks 1 and 24, respectively; treatment effect was rated “worse than at the last visit” by 13% (8 of 64) and 11% (4 of 37) participants at weeks 1 and 24, respectively.
Conclusions
- The mean pain scores improved by 2 points at 1 weeks and reached meaningful clinically relevant levels after 8 weeks and scores continued to improve throughout follow-up;
- 92% (59 of 64) patients achieved pain palliation;
- Opioid doses were stabilised, and functional status was maintained over 6 months;
- Quality of life improved over the course of the study period.
Overall, the data shows a rapid and durable pain relief along with a decrease in MEDD* and a corresponding increase in the quality of life for patients with bone metastases.
Cryoablation offered an alternative to opioids for pain control.
*MEDD = Morphine Equivalent Daily Dose
Most participants achieved their maximum palliation by
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
TheraSphere is a registered trademark of Theragenics Corporation, used under license by Boston Scientific Medical Device Limited, a wholly owned indirect subsidiary of Boston Scientific Corporation. Simplicit90Y is a registered trademark of Boston Scientific Medical Device Limited. Simplict90Y is developed by Mirada Medical Ltd. and used under license by Boston Scientific Corporation, the sales agent for Simplicit90Y. All rights reserved.
















