DOSISPHERE-01 trial summary
This Level 1 clinical trial demonstrated that personalised TheraSphere dosimetry, using multicompartamental dose administration, achieved a 26.6 month median OS for large tumour HCC patients and a 16-month survival improvement compared to the control arm on standard dosimetry.
Garin E, Tselikas L, Guiu B, Chalaye J et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2020; Published Online: November 06, 2020 https://doi.org/10.1016/S2468-1253(20)30290-9
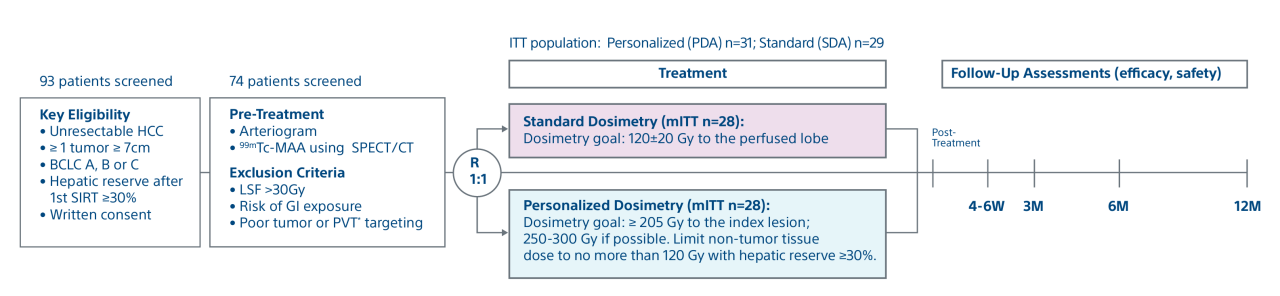
Study objectives and design
A randomised, multicentre, investigator sponsored phase 2 trial comparing the clinical outcomes of SIRT with TheraSphere in patients with advanced HCC using two pre-treatment dosimetry determination methods: (1) Standard, single-compartment dosimetry (STD); defined as a uniform distribution of absorbed dose within the perfused volume – both tumour and normal liver or (2) Personalised dosimetry (PERSO); defined as multi-compartment Y90 distribution of absorbed dose within the perfused volume that accounts for preferential blood flow into the tumor compared with normal parenchyma.
Study design
Key results
Personalised dosimetry improved survival
Median overall survival in the intention-to-treat (ITT) population
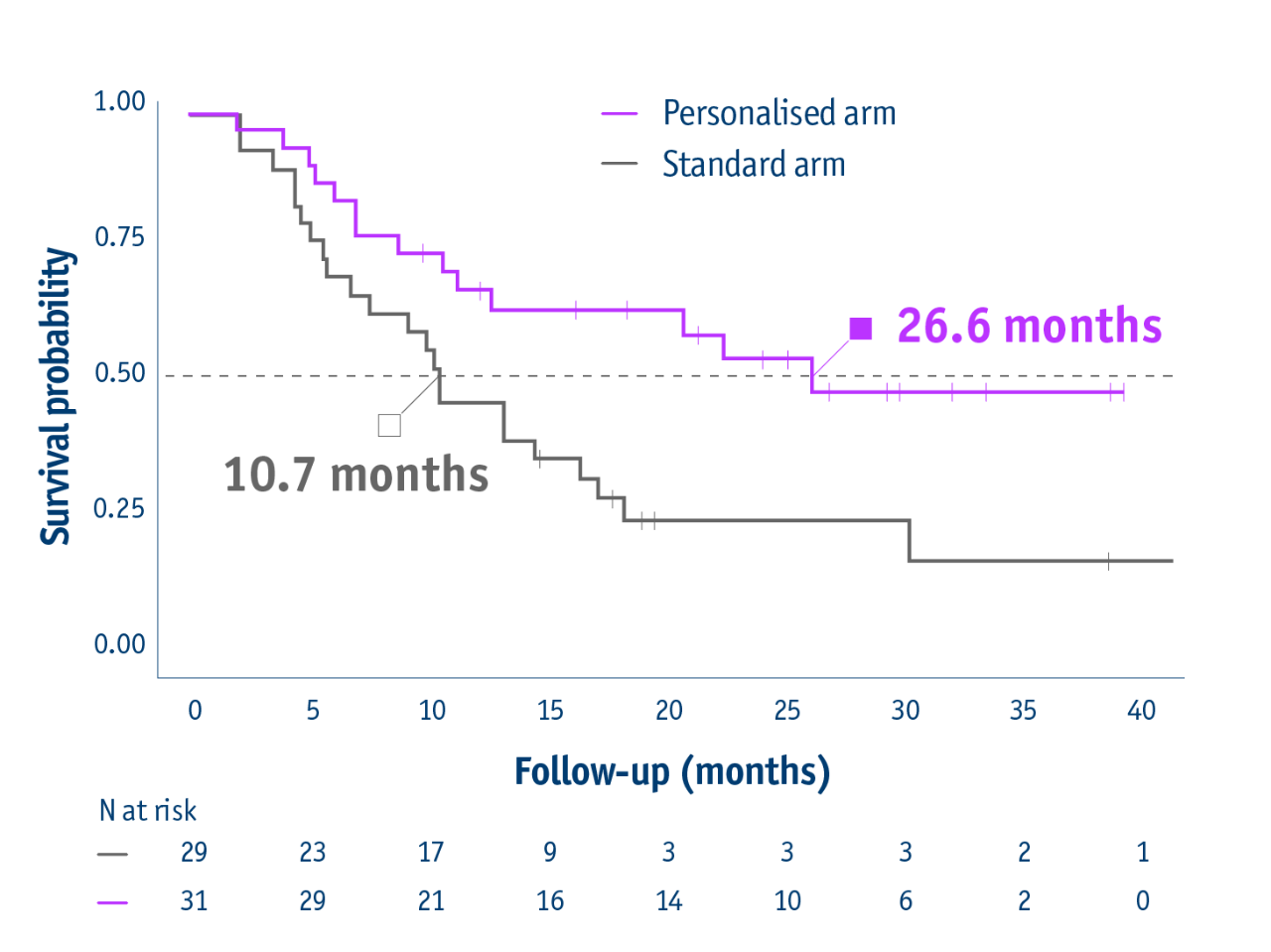
- 26.6 months median OS with personalised dosimetry
(95% CI: 11.7–not reached) - 10.7 months median OS in the standard dosimetry group
(95% CI: 6.0–16.8)
HR: 0.421 (95% CI: 0.215–0.826, P=0.0096)
23 months median OS for PVT patients in personalized arm vs 9.5 months in standard arm
16-month survival improvement
Personalised dosimetry improved response
Index lesion response rate at 3 months using EASL in mITT population
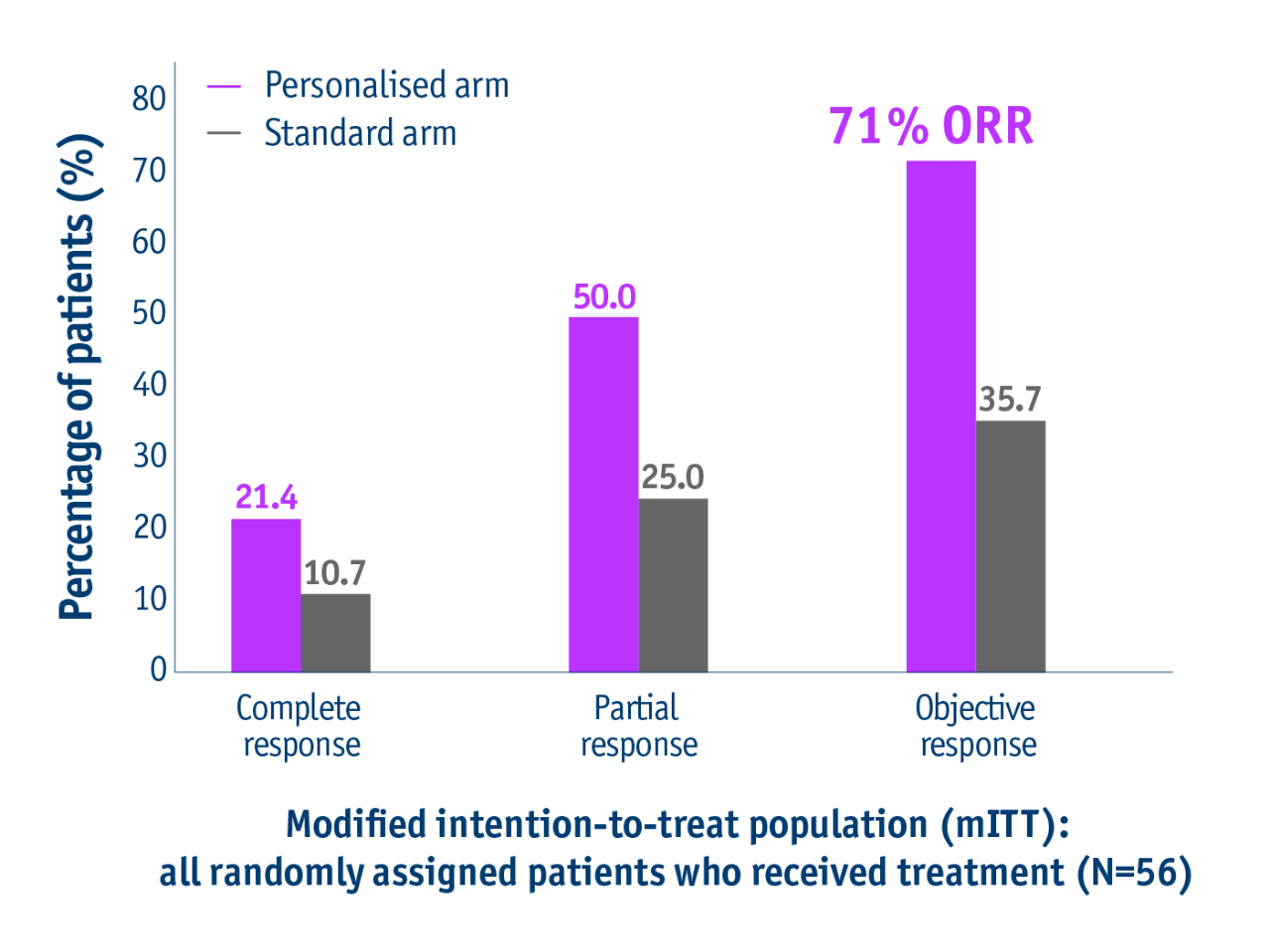
- Doubled response rates in patients with unresectable locally advanced HCC following personalised TheraSphere Y90 Therapy vs standard dosimetry2
Personalised dosimetry allowed more patients to be downstaged to surgery
Overall patient population downstaged
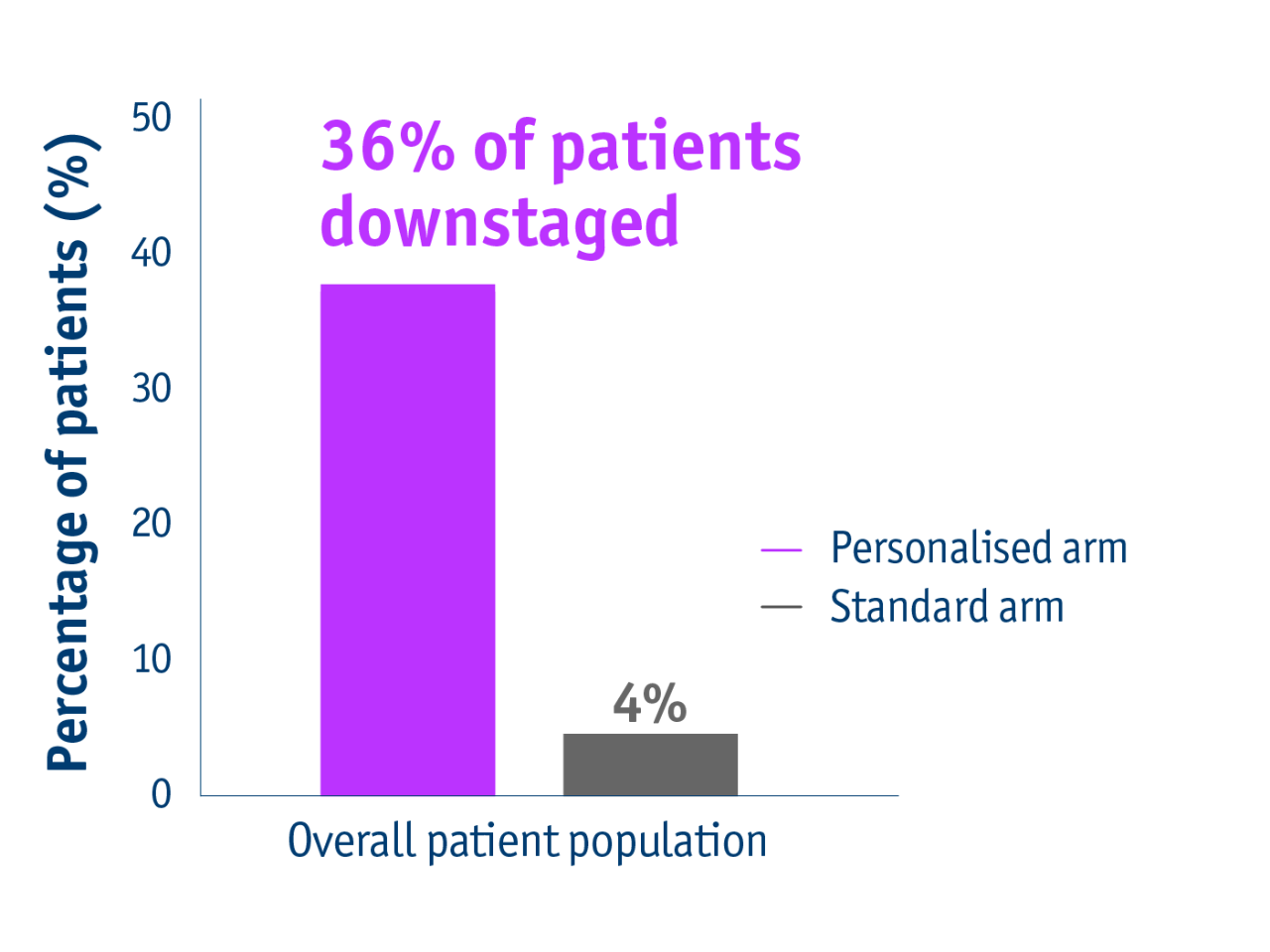
- 36% of patients with unresectable locally advanced HCC accessed liver resection following personalised TheraSphere Y90 Therapy vs standard dosimetry2
PVT patient population downstaged
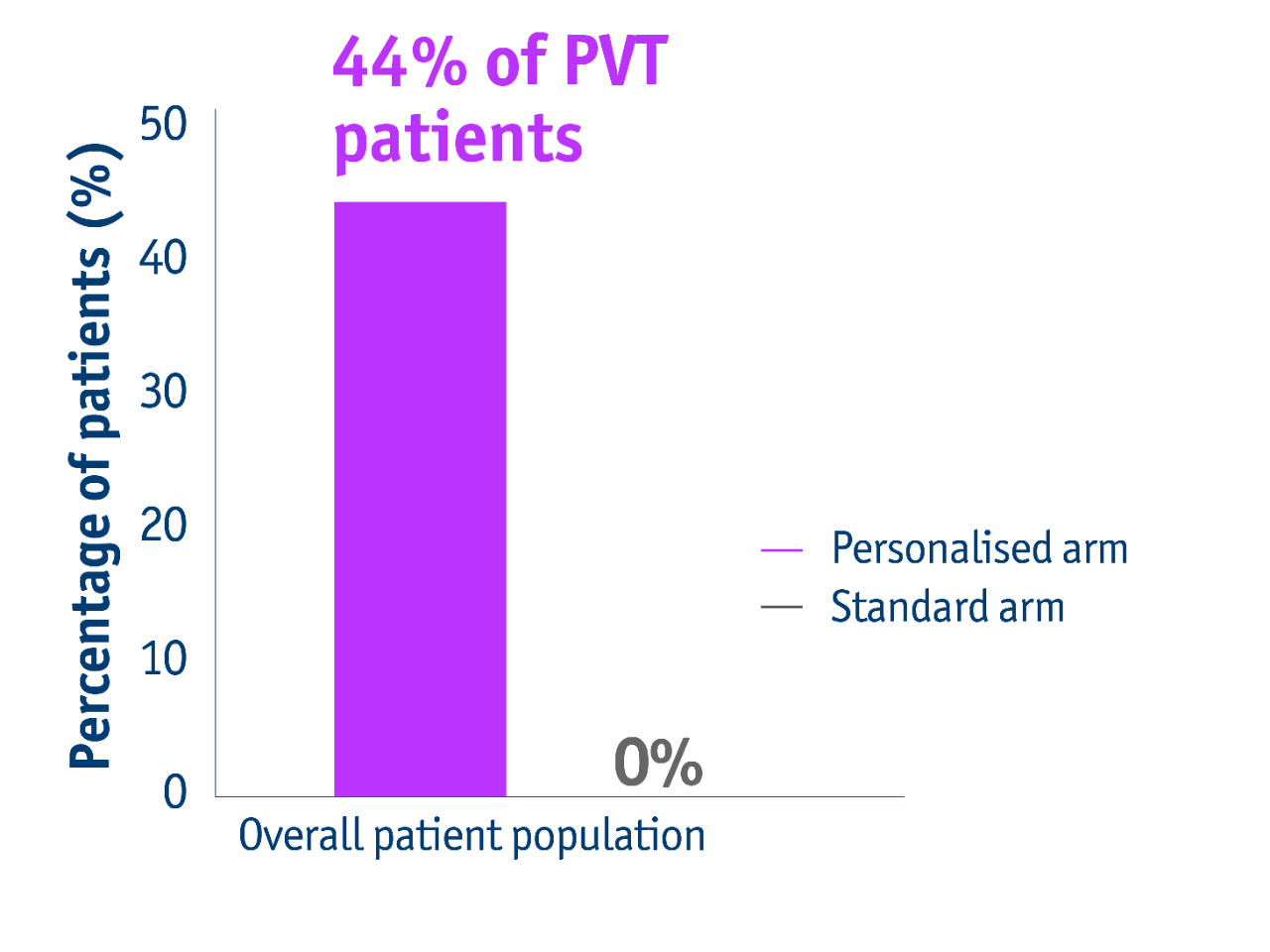
- 44% of PVT patients with unresectable locally advanced HCC accessed liver resection following personalised TheraSphere Y90 Therapy vs standard dosimetry2
Robert J Lewandowski, MD, Riad Salem, MD, DOSISPHERE Editorial, Lancet Gastroenterology & Hepatology
Patient demographics (mITT population)
| Parameter | PDA (n = 28) | SDA (n = 28) |
| Male (%) | 92.9 | 92.9 |
| Child-Pugh Status (%) | CP A5: 786 CP A6/B7: 21.4 | CP A5: 786 CP A6/B7: 21.4 |
| BCLC (%) | BCLC B = 11 BCLC C = 89 | BCLC B = 7 BCLC C = 93 |
| Bilobar (%) | 43 | 57 |
| Mean Total Biliburin (μM/L±S/D) | 14.0±6.4 | 14.3±6.4 |
| PVT* Present (%) | 64.3 | 75.0 |
| PVT° Location (%) | Segmental 29.6 Main/Lobar 30/33 | Segmental 32.1 Main/Lobar 32/43 |
| Index lesion (mean, cm) | 10.5±2.4 | 10.9±2.57 |
*TheraSphere not indicated for patients with PVT
Data in parenthesis are 95% CIs.
Treatment characteristics and dosimetry (mITT population)
| Investigator Assessments | PDA (n = 28) | SDA (n = 28) | P value |
| Number of Y-90 glass microspheres treatments | One treatment, n=26 Two treatments, n=2 | One treatment, n=23 Two treatments, n=5 | no (not significant) |
| Activity administered GBq (mean, min-max) | 3.6 (2.4-4.8) | 2.6 (2.2-3.0) | 0.0049 |
| AD* to perfused liver (Gy) Mean (±SD) | 178.4±59.9 | 120.3±15.2 | 0.0001 |
| % of patients with AD to perfused liver > 150 Gy | 68 | 4 | <0.0001 |
| AD to index lesion (Gy) Mean (±SD) | 331.1±131.5 | 221.3±139.4 | 0.0007 |
| % of patients with AD to index lesion > 205 Gy | 88 | 38 | <0.0008 |
| AD to perfused normal tissue (Gy) Mean (±SD) | 92.8±30.1 | 64.5±36.6 | 0.007 |
*AD=absorbed dose
Liver adverse events (Grade ≥3) related to TheraSphere Y90 Therapy*
| Liver adverse events (Grade ≥3) related to TheraSphere Y90 Therapy* | ||
| Personalised (n=35) | Standard (n=21) | |
| Patients with ≥ 1 AE | 3 (8.6%) | 3 (14.3%) |
| Death | 1 (2.9%) | 1 (4.8%) |
| Liver AEs | 4 (11.4%) | 5 (23.8%) |
| • Ascites | 1 (2.9%) | 2 (9.5%) |
| • Encephalopathy | 0 | 0 |
| • GI haemorrhage | 0 | 2 (9.5%) |
| • Bilirubin increase/jaundice | 1 (2.9%) | 2 (9.5%) |
| • Hepatic failure | 2 (5.7%) | 0 |
*Adverse events occurring in >5% of patients
DOSISPHERE-01 conclusion
References
1.Gabr A, al. Liver Transplantation Following Yttrium‐90 Radioembolization: 15‐year Experience in 207‐Patient Cohort. Hepatology 2021;73(3):998-1010.
2.DOSISPHERE-01: Garin E, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021 Jan;6(1):17-29.
Abbreviations
AE, adverse event; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; GI, gastrointestinal; HCC, hepatocellular carcinoma; HR, hazard ratio; mITT, modified intention-to-treat; ORR, objective response rate; PVT, portal vein thrombosis; Y90, yttrium-90.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
TheraSphere is a registered trademark of Theragenics Corporation, used under license by Boston Scientific Medical Device Limited, a wholly owned indirect subsidiary of Boston Scientific Corporation. Simplicit90Y is a registered trademark of Boston Scientific Medical Device Limited. Simplict90Y is developed by Mirada Medical Ltd. and used under license by Boston Scientific Corporation, the sales agent for Simplicit90Y. All rights reserved.
















