LEGACY study overview
The LEGACY study is a robust multicentre study confirming TheraSphere as a neoadjuvant or standalone therapy in treating HCC1.
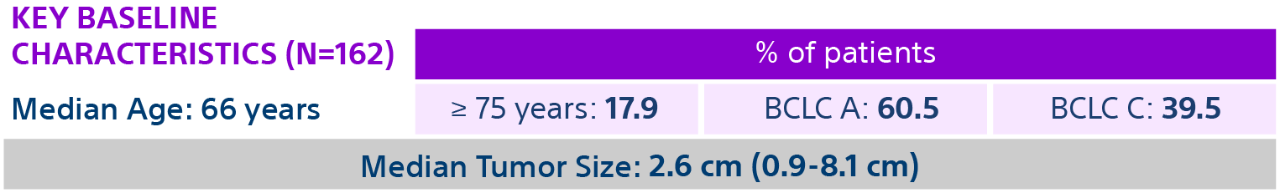
It included 162 consecutive treated patients with HCC. Presented by Riad Saleem, MD at CIRCE 2020.

For health care professionals in EUROPE except those practicing in France as the following pages are intended to all International health care professionals and are not in compliance with the French Advertising law N°2011-2012 dated 29th December 2011 article 34. Other health care professionals should select their country in the top right corner of the website.
Please note that the following pages are exclusively reserved for health care professionals in countries with applicable health authority product registrations. To the extent this site contains information, reference guides and databases intended for use by licensed medical professionals, such materials are not intended to offer professional medical advice. Prior to use, please consult device labeling for prescriptive information and operating instructions.
This Website is protected by the laws on copyright and by the relevant international conventions. It is strictly forbidden to make copies, whether partial or total and on whichever media without prior approval.
The LEGACY study is a robust multicentre study confirming TheraSphere as a neoadjuvant or standalone therapy in treating HCC1.
It included 162 consecutive treated patients with HCC. Presented by Riad Saleem, MD at CIRCE 2020.
To assess local tumour control and duration of response following treatment with Y-90 glass microspheres in patients with unresectable solitary HCC lesions.
100%
Patients in the LEGACY study responded to TheraSphere treatment(s)
93%
Overall Survival rate in patients with transplant or resection following TheraSphere at 3 years
88%
Demonstrated rate of best response by TheraSphere
(Treated population)
93% overall survival rate in patients with transplant or resection following TheraSphere at 3 years

(Best response in evaluable population, localised mRECIST)
88% demonstrated rate of best response by TheraSphere (i.e. CR, PR) by localised mRECIST.

Multicentre, single-arm, retrospective study conducted at 3 U.S. sites.* Consecutive patients meeting the eligibility criteria were treated with TheraSphere Y-90 Glass Microspheres at each site between January 2014 and December 2017.
Unresectable solitary lesions (≤ 8 cm); Selective, lobar, or mixed administration of Y-90 glass microspheres (TheraSphere); Treatment purpose (neoadjuvant to transplantation or resection or stand-alone treatment); Child-Pugh score A; BCLC A or BCLC C (ECOG 1); No prior liver transplantation, resection, locoregional treatment or systemic therapy; No portal vein thrombosis or extrahepatic disease.
Determined by Blinded Independent Central Review (BICR)
72.2%
(n=117/162)
Objective Response Rate (ORR) defined as CR or PR using localized mRECIST (defined as the response within the Y-90 glass microsphere treatment area) with confirmation of response (>4 weeks).
76.1%
(n=89/117)
Duration of Response (DoR) using localised mRECIST.**
Safety: Majority of adverse events were mild and resolved without medical intervention.
LEGACY is the first multicentre study to report a high median perfused volume absorbed dose of 410 Gy with TheraSphere, which resulted in an 88% best response, excellent and durable tumour control and high overall survival rate in patients with early and advanced HCC.
HCC: hepatocellular carcinoma; BCLC: Barcelona Clinic Liver Cancer staging system; Y-90: Yttrium-90; Gy: Gray; IQR: Interquartile Range; CR: Complete Response; PR: Partial Response.
* University of Washington, Seattle, WA; Northwestern University, Chicago, IL; Mount Sinai Health System, New York, NY.
** Median follow-up was 29.9 months [95% CI: 24.7, 34.6]..
TheraSphere is a registered trademark of Theragenics Corporation used under license by Boston Scientific Medical Device Limited, a wholly owned indirect subsidiary of Boston Scientific Corporation.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.