Medical Specialties > Interventional Radiology > Ablation Solutions > Ablation Products
Ablation Products
ICEfx™ Cryoablation System
The ICEfx™ Cryoablation System the next generation of ablation systems with a compact design and interface that offers predictable, reliable performance with seamless therapy delivery and exceptional ease of technical operation.
Learn more about ICEfx™
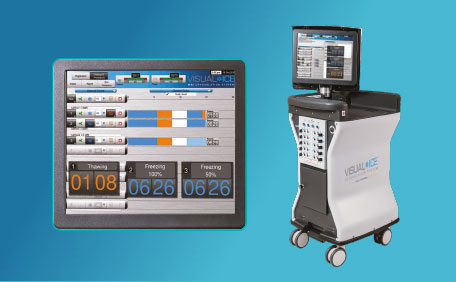
Visual-ICE™ Cryoablation System
The Visual-ICE™ Cryoablation System is intended for the cryoablative destruction of tissue during minimally invasive percutaneous procedures. It provides superior freezing performance, an intuitive user interface and innovative platform that incorporates helium-free options for thawing and cautery for track ablation.
Learn more about Visual-ICE™
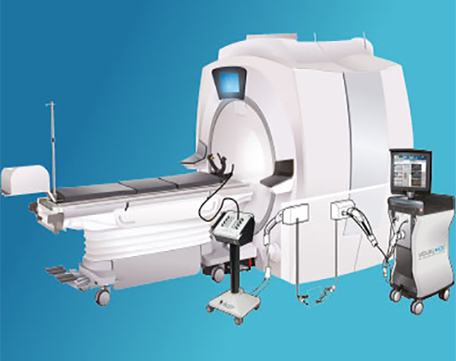
Visual-ICE™ MRI Cryoablation System
Specialised for the magnet room, the Visual-ICE MRI Cryoablation System is the only ablation system that leverages the unique advantages of cryoablation zone visibility with the exquisite image resolution of MR. The Visual-ICE MRI offers a safe and efficient cryoablation procedure facilitating precise and effective treatment, without the need for surgery or repeated radiation treatments.
Learn more about Visual-ICE™ MRI
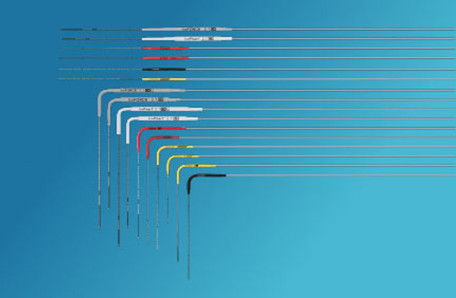
Cryoablation Needles
Boston Scientific’s comprehensive cryoablation portfolio includes the narrowest gauge needles available today, allowing precise iceball shaping while minimising bleeding. They are also very easy to control!
Radiofrequency Ablation
Designed for use with the RF3000™ Radiofrequency Generator
- Umbrella-shaped array
- Wide portfolio matrix of array diameters from 2cm to 5cm
- Soloist Single Needle Electrode for small 1.5cm x 1cm ablation zones
- The only RFA system that utilizes impedance to accurately assess the procedural endpoint
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, Warnings and instructions for use can be found in the product labelling supplied with each device. Information for the use only in countries with applicable health authority product registrations. Material not intended for use in France.