Clinical Studies

New data from the EWOLUTION 1 year registry: WATCHMAN™ LAAC Device is effective in reducing the risk of stroke

One-year data from the EWOLUTION registry confirmed that the WATCHMAN™ LAA Closure device had a high implant success rate and was effective in stroke reduction for patients with nonvalvular atrial fibrillation. It also found that LAAC with WATCHMAN™ followed by dual antiplatelet therapy significantly reduced the risk of stroke and lowered the risk of major bleeding by more than half as compared to Warfarin use.
SCAAR Registry from EuroPCR 2017: SYNERGY™ Stents presents very low definite ST rates
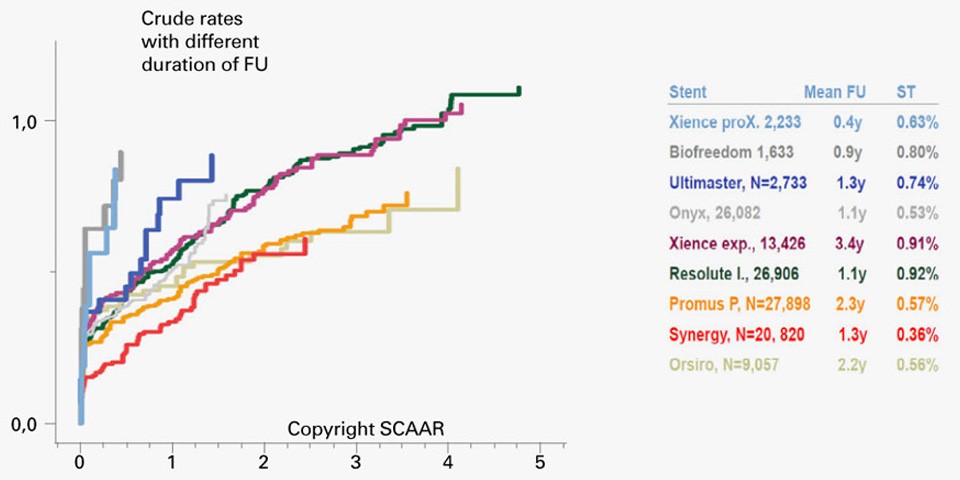
SYNERGY™ Stent presents best in class healing and is associated with low cardiac events in complex high risk indicated patients (CHIP). This is the result of the SCAAR Registry data showed during EuroPCR 2017.
Latest updates from EuroPCR 2017: the EVOLVE II Diabetes Substudy
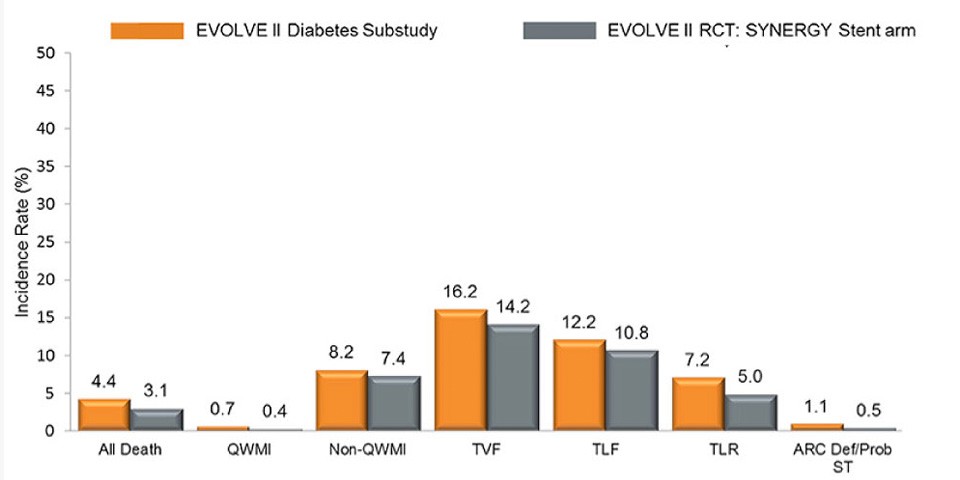
EVOLVE II Diabetes Substudy is a consecutive, multicenter, single-arm, non-randomized study with the SYNERGY™ Stent in medically-treated diabetic patients. SYNERGY™ BP Stent showed excellent performance as compared to all patients in the EVOLVE II Trial at three years:
- 1.1% def/Prob Stent Thrombosis at 3 years
- Patients with DM treated with the Synergy™ BP Stent had 0% definite/probable ST after 30 days
LOTUS™ Valve: REPRISE III 1 Year data from EuroPCR 2017

LOTUS™ Valve showed superiority to CoreValve® TAVI System Platform in the primary effectiveness endpoint at 1 year and non-inferiority in the primary safety endpoint at 30 days. This is the result of the REPRISE III 1 year data trial. Watch the REPRISE III data highlights animation and read the full deck presented by Dr. T. Feldman at EuroPCR 2017.














