Congresses and Events

Boston Scientific announces long-term data from the EVOLVE Trial of the SYNERGY™ Stent presented at EuroPCR 2016
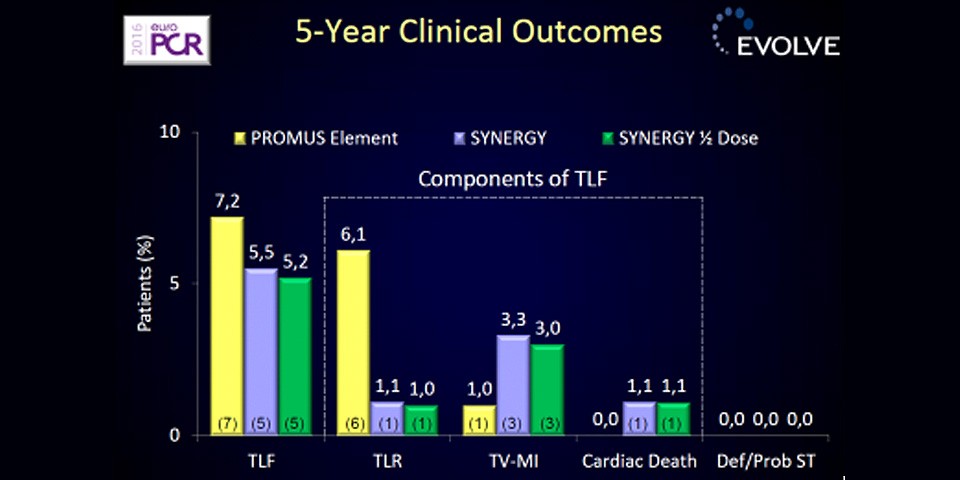
The final 5-year results of the EVOLVE Trial reinforce earlier findings of sustained performance of the SYNERGY™ Stent, with no stent thrombosis, a very low target lesion revascularisation rate (1.1%) and no significant differences between SYNERGY™ and control for the key endpoints of target lesion failure, cardiac death, or myocardial infarction.
SYNTAX II 30-Day Results: FFR / iFFR and IVUS-guided percutaneous coronary revascularisation with new-generation DES in patients with De Novo three vessel disease

SYNERGY performed very well in SYNTAX II. FFR/iFFR and IVUS Guided PCI for multivessel coronary disease with the SYNERGY Stent (SYNTAX II strategy) results in statistically significant lower MI and ST rates at 30 days when compared to the control of the SYNTAX I trial.
Boston Scientific announces new data from the RESPOND study of the LOTUS™ Valve presented at EuroPCR2016

Results from the RESPOND Study evaluating the Boston Scientific LOTUS™ Valve demonstrated excellent outcomes of key safety and efficacy endpoints through 30 days post implant procedure. The data show excellent device performance, a strong safety profile and extremely low rates of paravalvular leak.
New data from the EWOLUTION registry presented at EuroPCR 2016 confirms safety of the Boston Scientific WATCHMAN™ Left Atrial Appendage Closure device

The 3-month results from the EWOLUTION Registry on WATCHMAN™ outcomes in Real-Life Utilization found that LAA closure with the Boston Scientific WATCHMAN™ device has a high success rate in complete LAA closure with low periprocedural risk, independently of implanting physicians’ experience, thus confirming the safety of the device.














