WATCHMAN FLX™
Left Atrial Appendage Closure Device
Built off the most studied, most implanted LAAC device in the world, WATCHMAN FLX is a proven alternative to long-term OAC therapy1 for stroke risk reduction in patients with non-valvular atrial fibrillation.
WATCHMAN FLX was studied in the PINNACLE FLX US IDE Clinical Trial. The study was designed to establish the procedural safety and closure efficacy of the next generation LAAC device, WATCHMAN FLX.
Videos
OPTION Randomized Clinical Trial
Surpass Early Results
Study Design
- SURPASS assesses the safety and efficacy outcomes in patients in the NCDR-LAAO Registry that received a commercial WATCHMAN FLX device
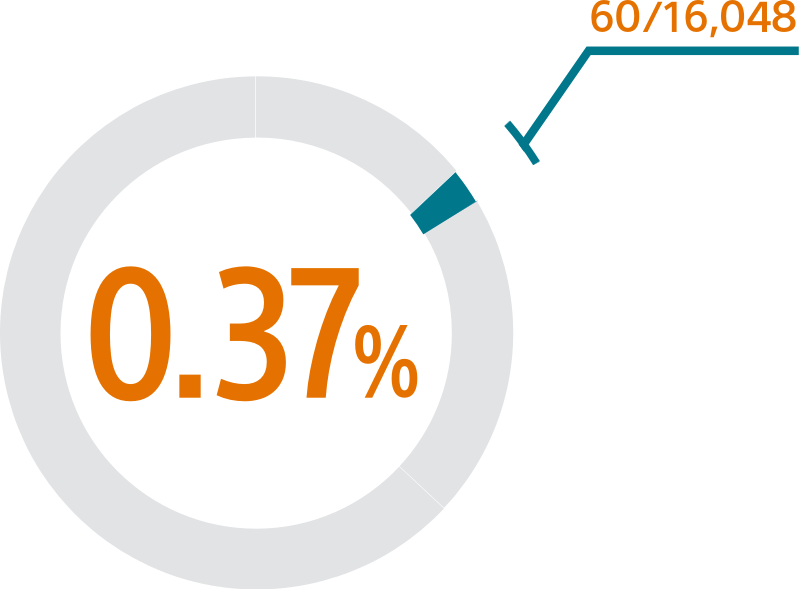
- This SURPASS analysis includes 16,048 patients receiving a WATCHMAN FLX device between August 5, 2020 and March 31, 2021
- SURPASS will continue to assess WATCHMAN FLX patients included in the NCDR-LAAO Registry from August 2020 through August 2022 and will follow these patients through 2 years post-implant.
Mean baseline patient characteristics (N=16,048)
- Average age/years: 76.1 ± 7.9
- CHA2DS2-VASc: 4.8 ± 1.5
- HAS-BLED: 2.4 ± 1.0
- 40% women
- 62% with prior clinically relevant bleeding event
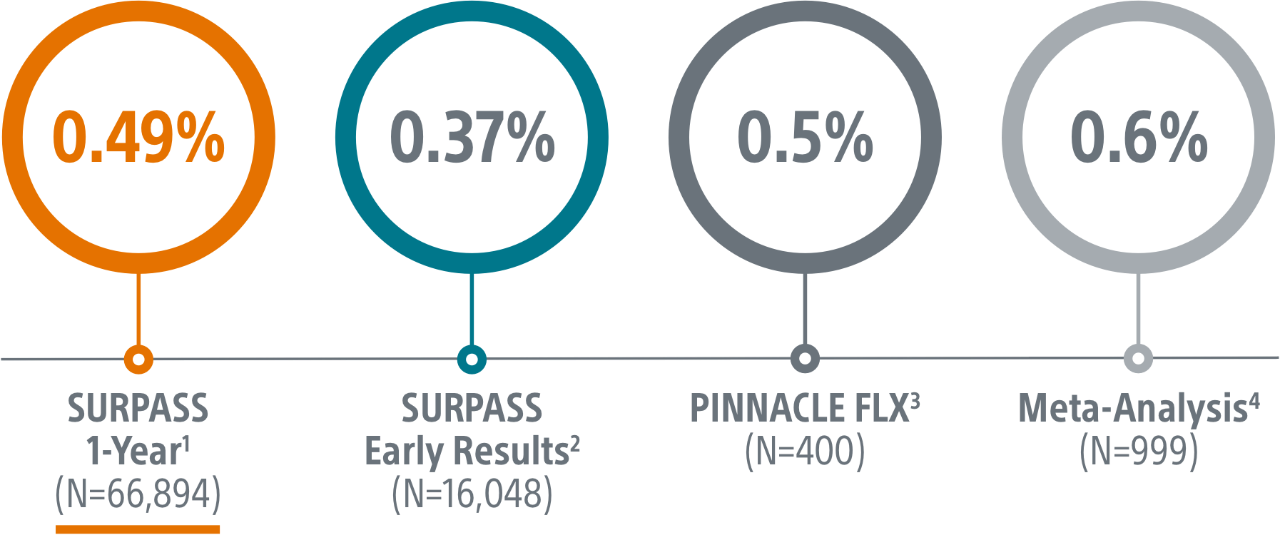
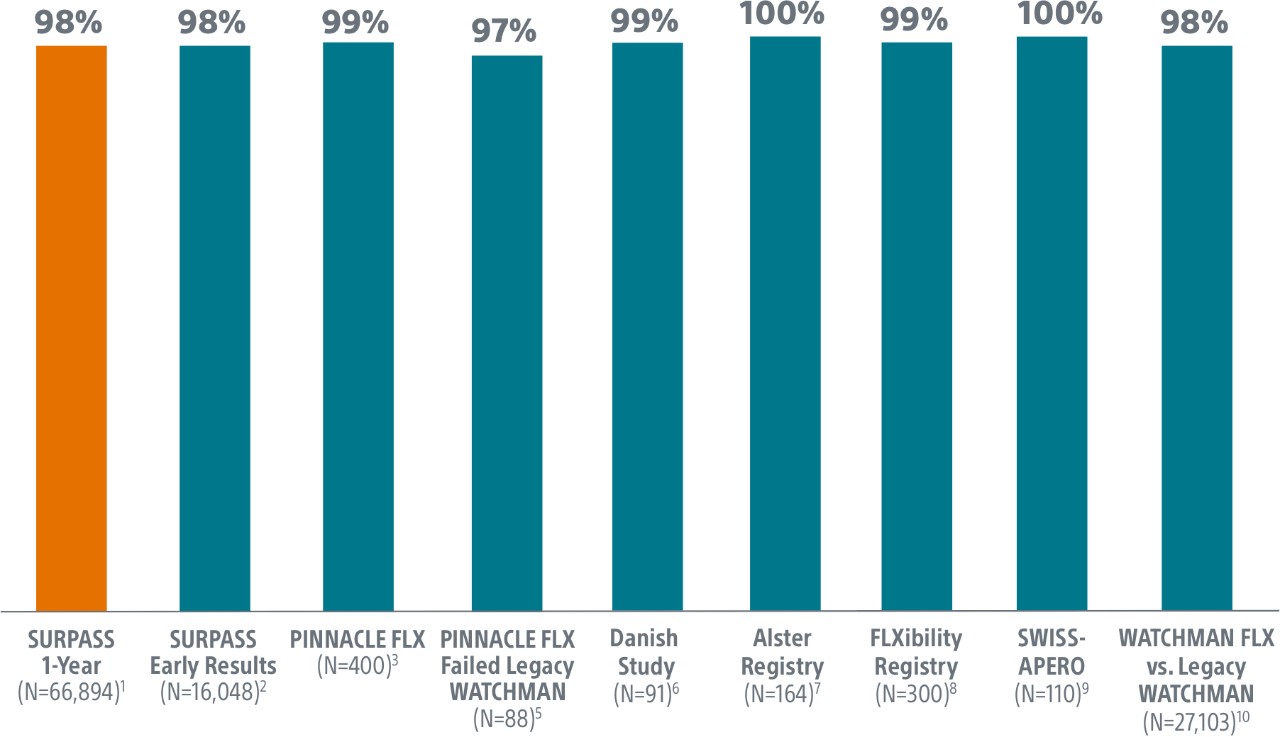
SURPASS demonstrated 0.37% major procedural adverse event rate within 7 days or hospital discharge in 16,048 commercial patients and confirmed the trusted safety profile of WATCHMAN FLX in real-world clinical practice setting.
The SURPASS Data reinforces the excellent safety profile WATCHMAN FLX demonstrated in the PINNACLE FLX Trial, with the largest (n=16,048) real-world WATCHMAN FLX patients studied to date.
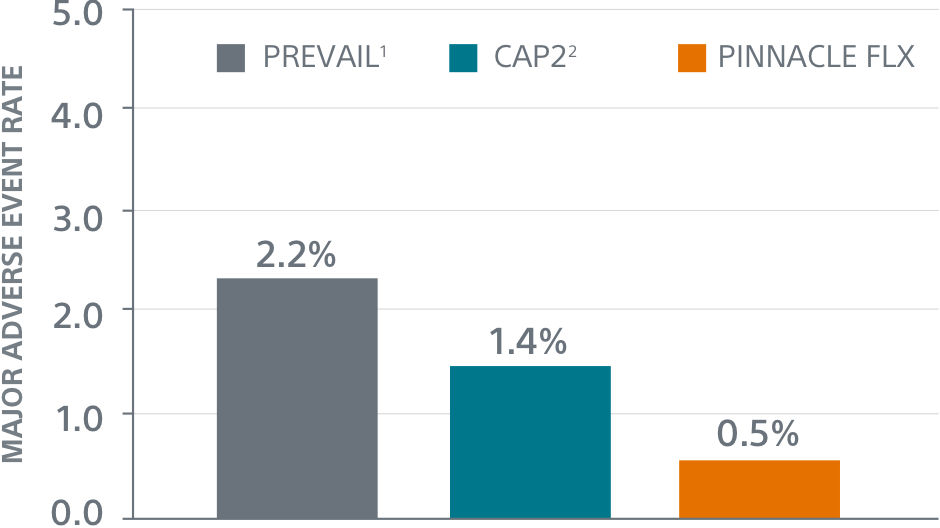
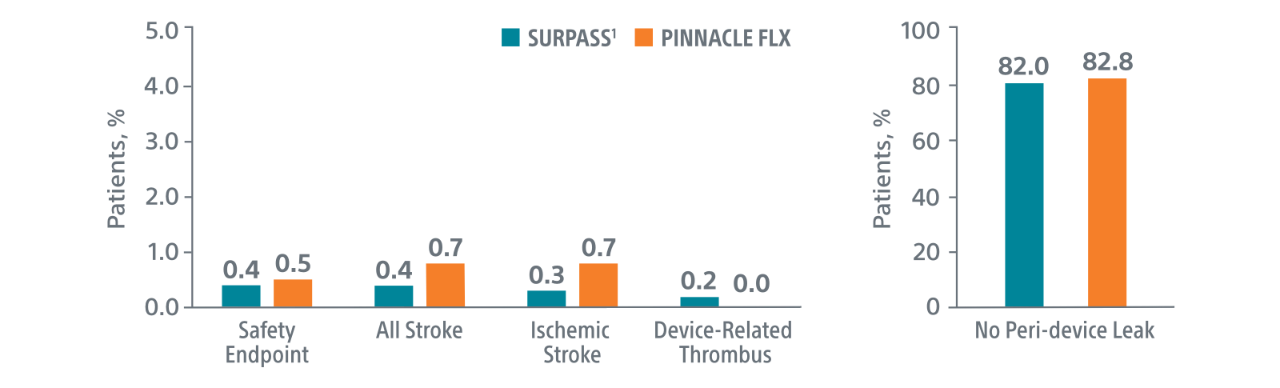
Comparison with PINNACLE FLX2
45-Day Outcomes
Surpass 1 Year Results
Study Design
- The objective of this SURPASS analysis is to assess long term safety and efficacy outcomes at one year with WATCHMAN FLX in a routine, real-world setting.
- This analysis includes the largest commercial WATCHMAN FLX patient population to date, with 66,894 patients implanted between August 5, 2020 and March 31, 2022.
Patient Characteristics:
- Age: 76.2 ±7.9 Years
- CHA2DS2-VASc Score: 4.8±1.5
- HAS-BLED Score: 2.4±1.0
- Women: 41%
- Clinically Relevant Bleeding: 57.7%

2. Kapadia, CRT 2022.
3. Kar, Circulation 2021.
4. Della Rocca et al. Heart Rhythm 2022.
5. Ellis, Heart Rhythm, 2021.
6. Korsholm, WM FLX First Experience, JACC, 2020.
7. Bergmann, Alster Registry, Presented ePCR 2021.
8. Betts, EHRA 2022.
9. Galea, SWISS APERO Trial, Cirulation, 2021.
10. Freeman, HRS 2022.
Meta-analysis
Study Design
- Meta-analysis (published in Heart Rhythm) of 4186 patients from 21 studies (a meta-analysis is a statistical technique used to systematically merge the findings of previous studies to calculate an overall or ‘absolute’ effect)
- 3187 Amulet implants
- 999 WATCHMAN FLX implants
- No difference in TE risk between groups
- CHA2DSC2-VASc: 4.3±1.5 for Amulet
- CHA2DSC2-VASc 4.2±1.5 for WATCHMAN FLX
- Data from a first imaging study performed within 3-month were used to assess the incidence of peri-device leaks >5mm and device-related thrombosis (DRT).
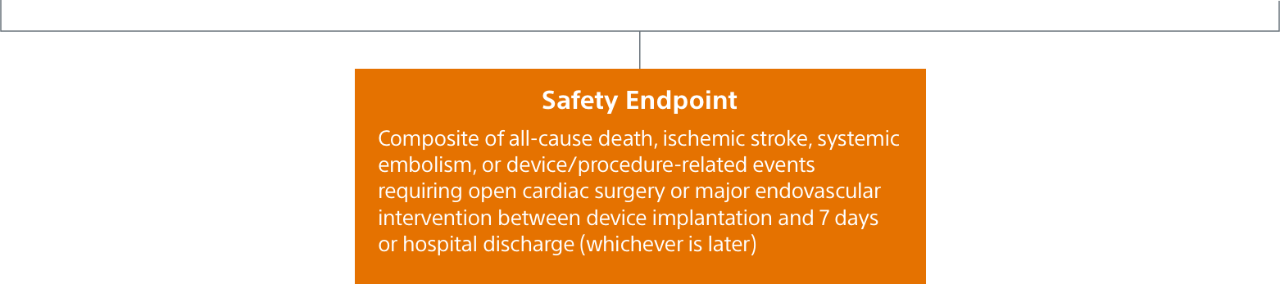
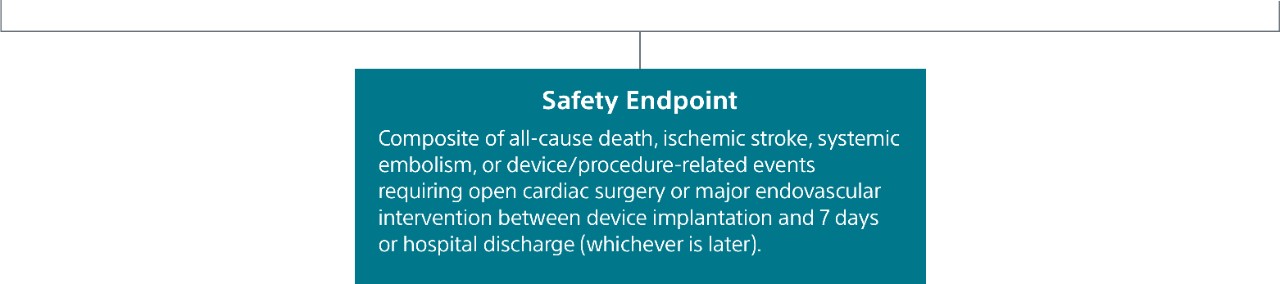
Safety Endpoint
Safety endpoint was the occurrence of death, stroke, major bleeding, myocardial infarction, major vascular complications, device embolization, or pericardial effusion within 7 days post-procedure.
Key Results
Safety
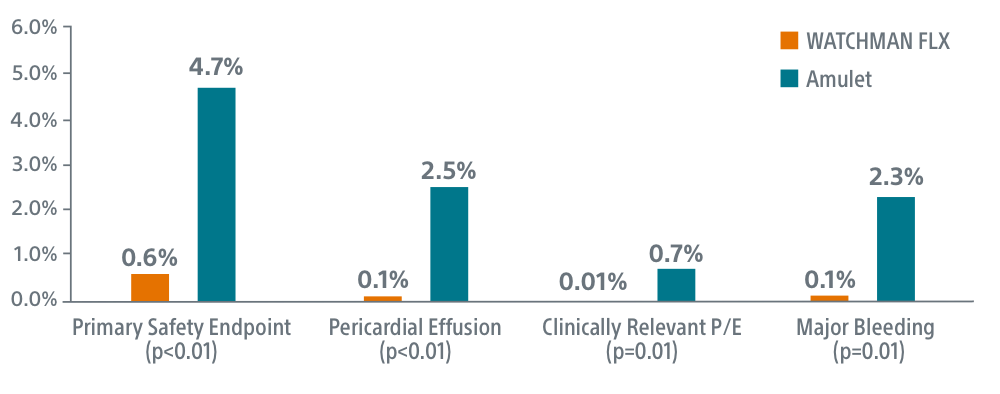
- WATCHMAN FLX superior on primary safety endpoint (0.6% FLX vs 4.7% Amulet, p<0.01)
- WATCHMAN FLX superior on overall pericardial effusion/tamponade (0.1% FLX vs 2.5% Amulet, p<0.01)
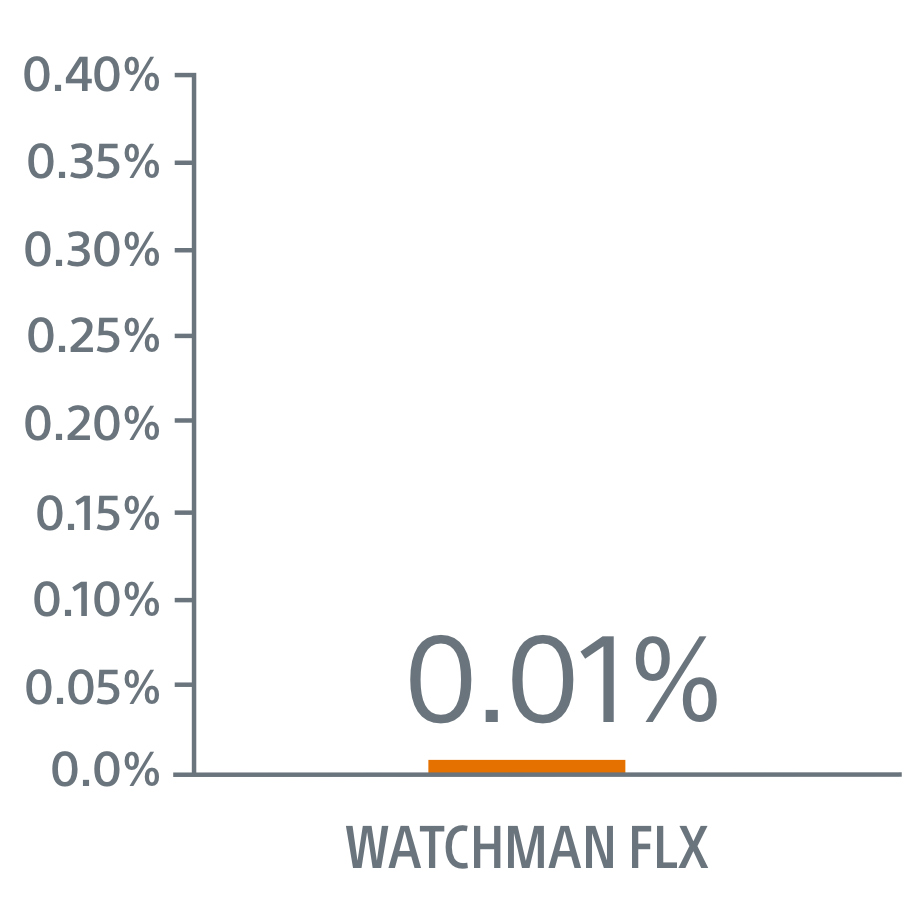
- WATCHMAN FLX superior on clinically relevant pericardial effusion/tamponade (0.01% FLX vs 0.7% Amulet, p=0.01)
- WATCHMAN FLX superior on peri-procedural major/intracranial bleeding (0.1% FLX vs 2.3% Amulet, p=0.01)
- 0 device embolizations occurred with WATCHMAN FLX vs. 15 with Amulet
- WATCHMAN FLX demonstrated lower DRT than Amulet (1% vs 1.6%)
- No difference was observed for death or stroke between groups
Simplicity
WATCHMAN FLX showed a trend towards higher procedural success (p=0.08). Procedural success was achieved in 99.9% of WATCHMAN FLX patients (99.4% for Amulet, P=0.08)
Seal
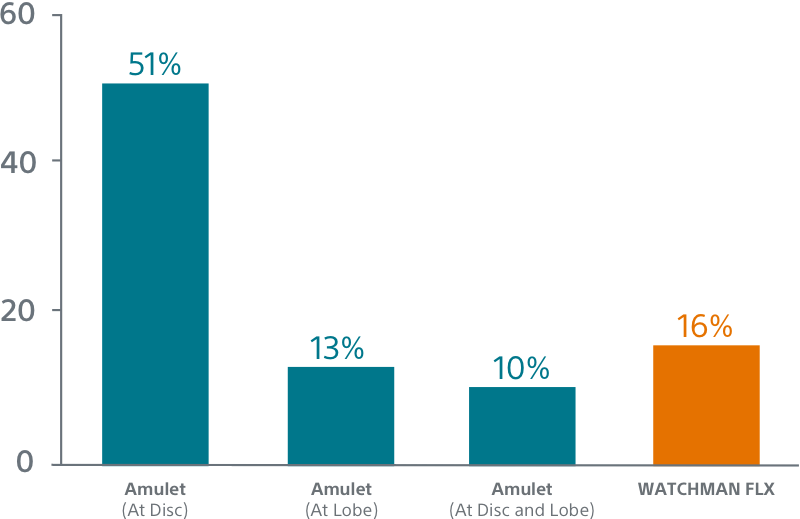
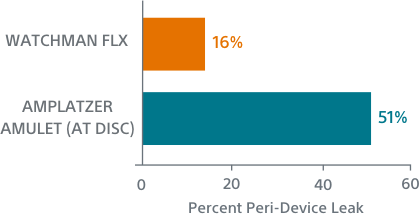
WATCHMAN FLX showed a trend towards more frequent appendage occlusion (leaks >5mm; p=0.06). WATCHMAN FLX demonstrated fewer peri-device leaks >5mm than Amulet (0.01% vs 0.34%, p=0.06)
Kapadia, SURPASS Registry, CRT 2022
SEAL FLX
Study Design
- Single-center, retrospective study of LAAO implantation at Aarhus
University Hospital (Denmark) between 2018-2020.
1st cohort: Amplatzer Amulet (n=150) 2018 – 2019
2nd cohort: WATCHMAN FLX (n=150) 2019 – 2020 - Cardiac CT was performed 8 weeks after LAAO
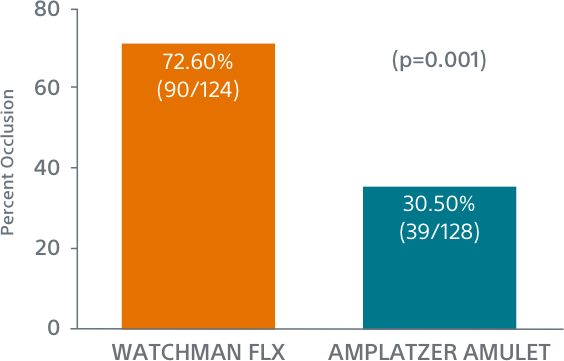
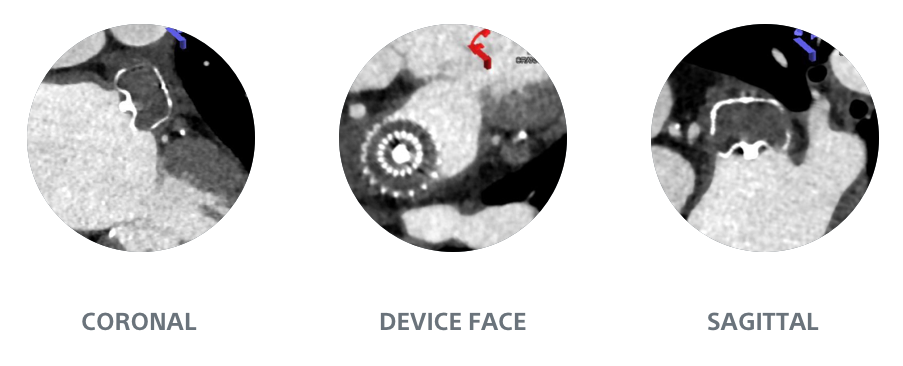
WATCHMAN FLX Demonstrated Statistically Superior Complete Occlusion** vs Amulet (p=0.001)
**Complete LAA occlusion defined as no visible peri-device leak (PDL) and absence of contrast patency in the distal LAA (LAA/left atrium Hounsfield ratio <0.25)
Simplified Sizing
WATCHMAN FLX

Single Landing Zone and Overlapping Treatment Ranges
AMPLATZER AMULET2
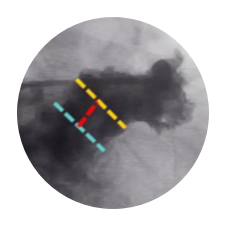
Complicated, Mis-Matched Measurements
Simplified Placement
WATCHMAN FLX
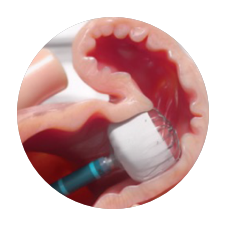
Precision Placement Control
AMPLATZER AMULET3
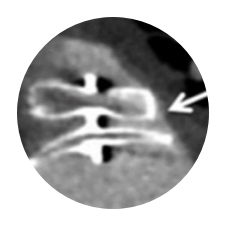
Trade-off Between Disc and Lobe Placement
Simplified Assessment
WATCHMAN FLX1
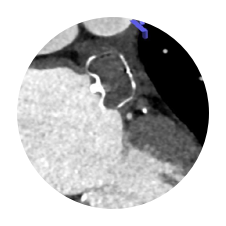
Full Visibility
AMPLATZER AMULET1
Hidden Leaks
2. Lakkireddy, Amulet Tips and Tricks in Simple and Complex Anatomy, TVT 2018.
3. Saw, Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure, European Heart Journal - Cardiovascular Imaging
SWISS-APERO
Comparison of Amulet vs Watchman/FLX Devices in Patients Undergoing Left Atrial Appendage Closure - SWISS-APERO
PINNACLE FLX

Study Design
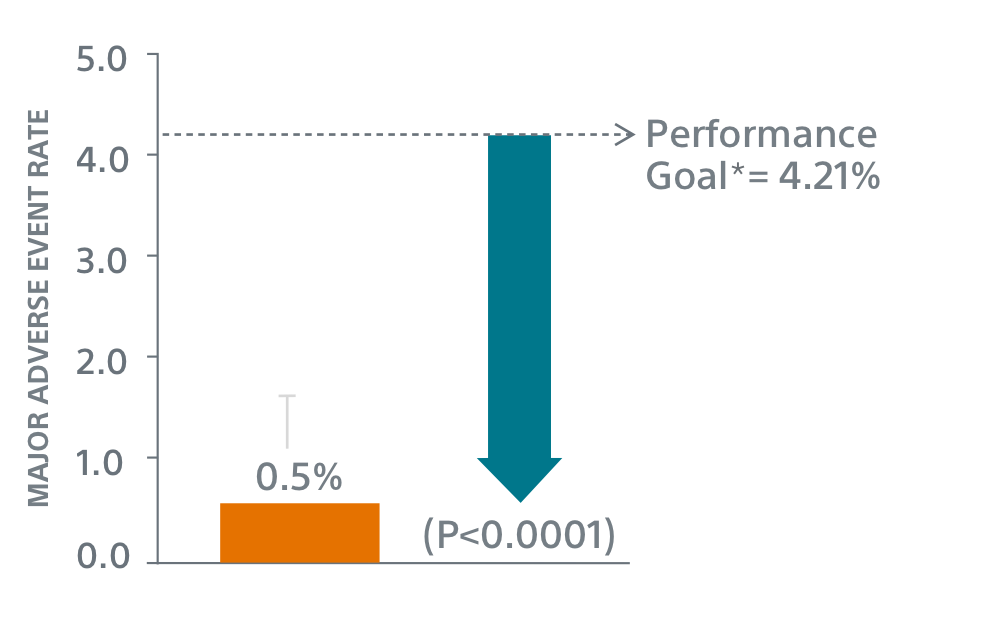
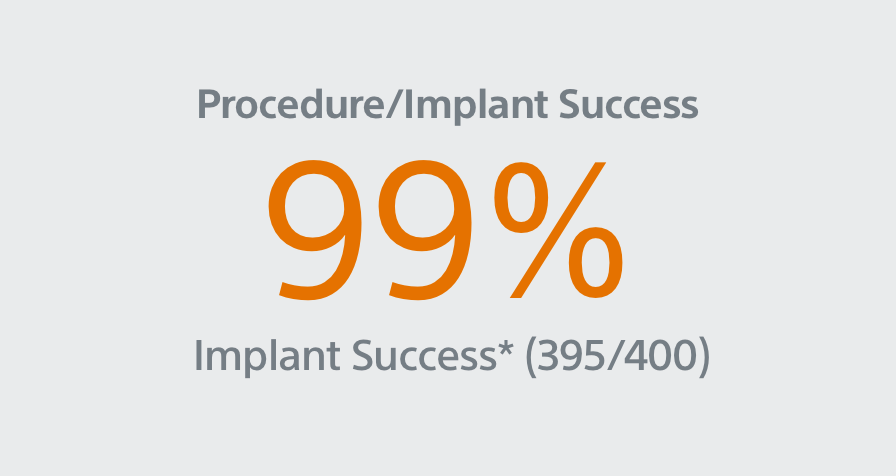
- 400 patient, 29 US site, single arm, non-randomized trial evaluating WATCHMAN FLX for non-inferiority to safety and efficacy performance goals based on the WATCHMAN™ device.
- Follow-up: 45 days (+TEE), 6 months, 12 months (+TEE), 18 months, and 24 months
- Patient Characteristics: Average CHA2DS2-VASc of 4.2±1.5, Average HAS-BLED of 2.0±1.0
- Post Implant Drug Regimen: NOAC/ASA for 45 days, Clopidogrel/ASA to 6 months, ASA post 6 months
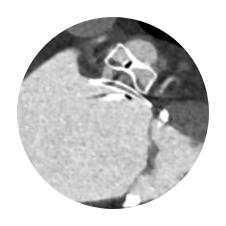
- Primary Safety Endpoint: All-cause death, ischemic stroke, systemic embolism, or device- or procedure-related adverse events requiring surgery or major endovascular intervention within 7 days following the procedure or by hospital discharge, whichever is later.
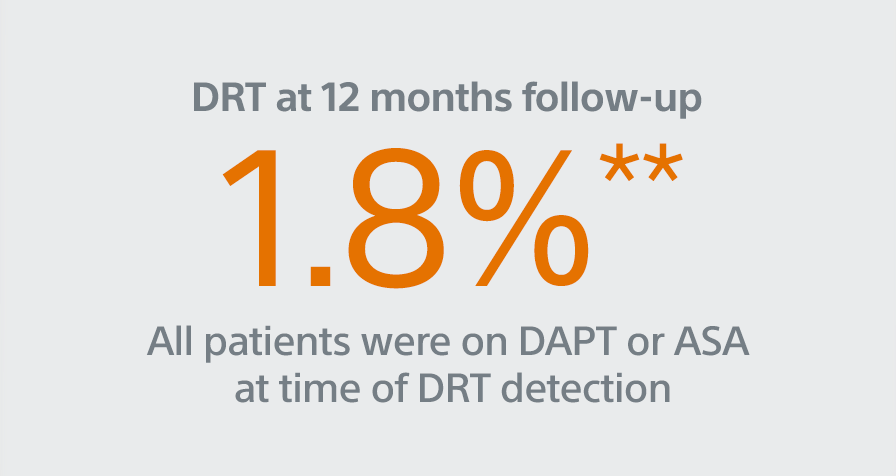
- Primary Efficacy Endpoint: The rate of effective LAA closure defined as any peri-device flow ≤5mm demonstrated by TEE at 12 months
- Secondary Efficacy Endpoint: The occurrence of ischemic stroke or systemic embolism at 24 months from the time of enrollment
- Inclusion/exclusion criteria is consistent with WATCHMAN clinical study inclusion/exclusion criteria. Patients must be eligible for short-term NOAC vs warfarin in previous clinical studies.
Primary Safety Endpoints
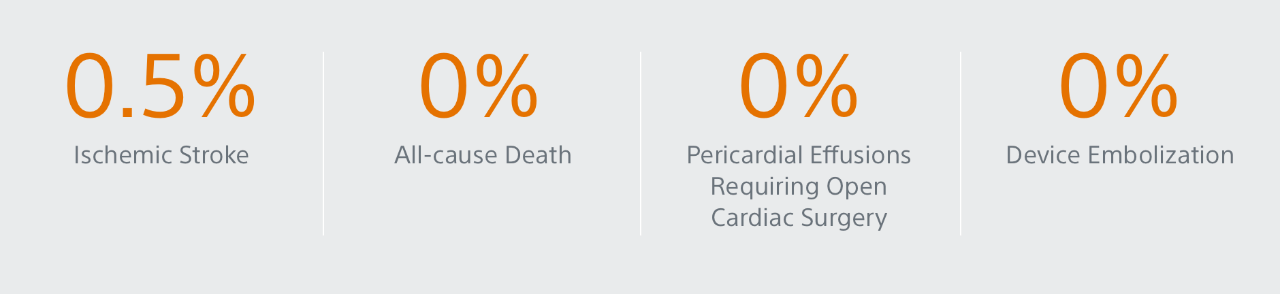
Primary Efficacy Endpoints
The rate of effective LAA closure defined as any peri-device flow ≤5mm demonstrated by TEE at 12 months.
** Performance goal pased on the rates observed in PREVAIL(1) and CAP2(2), minus a clinically relevant delta
Designed to Treat Widest Range of Patient Anatomies
WATCHMAN FLX sizes range from (20mm - 35mm)
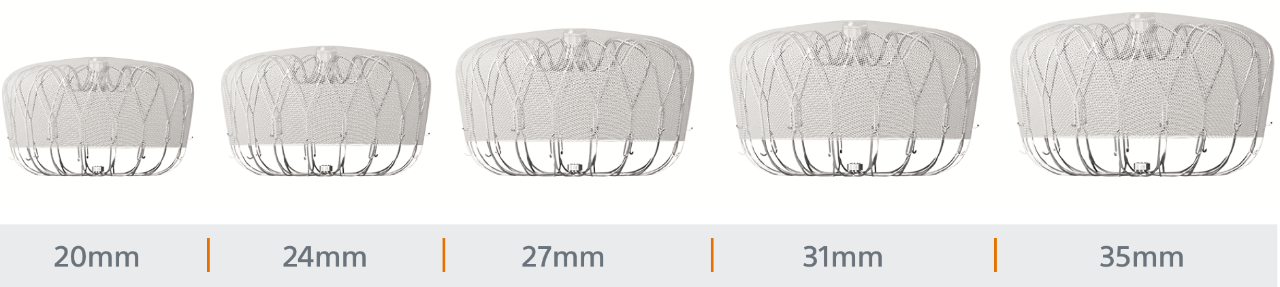
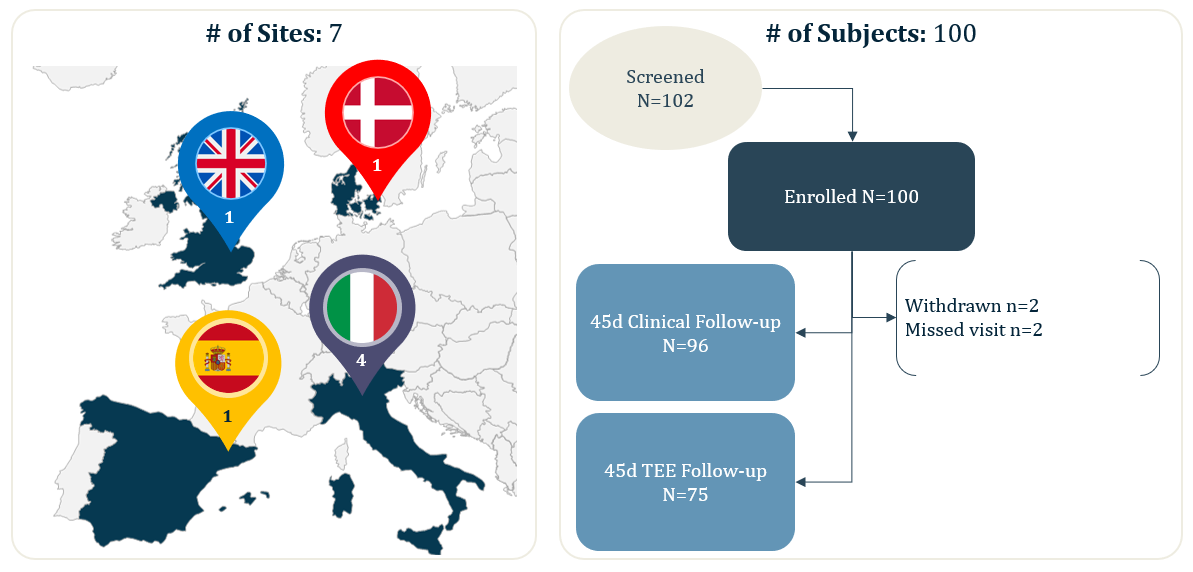
ICE LAA
Study Design
ICE LAA is a multi-center study with core-laboratory adjudication, this analysis reported the 45-days results from 100 real-world WATCHMAN FLX patients from 7 European centers.
Summary of the Results
- 100 real-world, European WATCHMAN FLX patients (from 7 implanting centers)
- 100% Device Success (Implantation of WATCHMAN FLX without in-hospital mortality)
- 100% Technical Success (Successful deployment and release, no conversion to TEE and effective closure of LAA at implant [no leak <5mm])
- 0% Conversion to standard TEE during implant
- 0% leak >5mm
- Key 45-days safety outcomes:
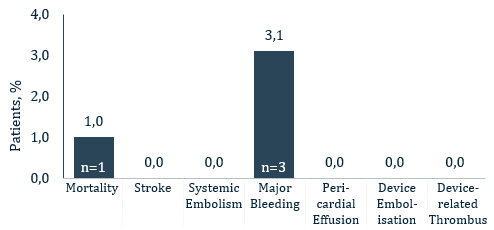
- 1% all-cause death
- 0% all stroke
- 0% pericardial effusion
- 0% DRT
- 0.% device embolization
- 3,1% major bleeding (BARC 3/5)
Outcomes with WATCHMAN FLX using Intracardiac Imaging: Results from ICE LAA
Jens Erik Nielsen-Kudsk
Aarhus University Hospital,
Aarhus, Denmark
Sergio Berti
Fondazione Toscana Gabriele Monasterio
Massa, Italy
Francesco Caprioglio
Ospedale San Bortolo
Vicenza, Italy
Frederico Ronco
Ospedale Dell Angelo
Mestre, Italy
Dabit Arzamendi
Hospital De La Santa Creu I Sant Pau
Barcelona, Spain
Timothy Betts
John Radcliffe Hospital
Oxford, UK
Claudio Tondo
Centro Cardiologico Monzino
Milan, Italy
Thomas Christen
Boston Scientific
Marlborough, MA USA
Dominic J Allocco
Boston Scientific
Marlborough, MA USA
Objective
The I Can sEe Left Atrial Appendage (ICE LAA) study will assess the use of ICE imaging of the LAA during WATCHMAN FLX implantation
Background
- The left atrial appendage (LAA) can vary greatly in volume and shape in different patients
- Transesophageal echocardiography (TEE) is the most commonly used diagnostic modality for visualizing the LAA during WATCHMAN implantation
- TEE carries some risk (e.g. aspiration, esophageal lesions) and typically requires a trained echocardiographer and general anesthesia.
- Not all patients are physically able to undergo general anesthesia and/or TEERecent single-center studies have suggested LAA closure under intracardiac echocardiography (ICE) guidance is feasible and safe.
- ICE LAA is a multi-center study with core-laboratory adjudication
Methods
Study Type: Prospective, non-randomized, single-arm, multi-center
| Age, years | 76 ± 8 | CHA2DS2-VASc score | 4.0 ± 1.5 |
| Female | 33% | HAS-BLED score | 2.5 ± 0.9 |
| Indication for LAAC | History of major or minor bleeding | 75% |
| History of major bleeding | 63% | |
| Increased risk for bleeding | 32% | |
| Inability to take OACs (not related to bleeding) | 23% | |
| Thromboembolic event and/or documented presence of thrombus in LAA despite adequate OAC therapy | 4% |
Results
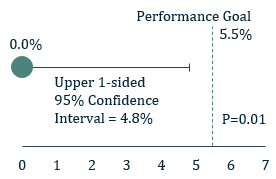
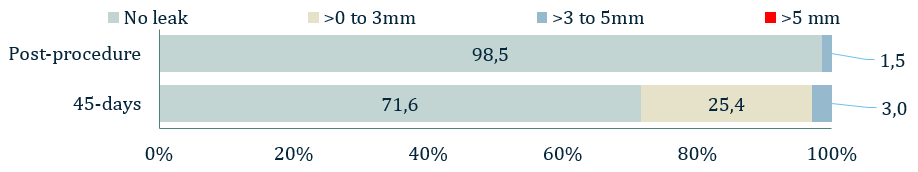
Figure 1: 1° Endpoint - Effective closure
- The 1° endpoint of ICE LAA was significant peri-device leak (>5 mm) based on the 45-day post-implant TEE and assessed by the echocardiographic core laboratory
- The 1° endpoint was met. The rate of leak >5 mm was 0.0% with an upper one-sided 95% confidence interval of 4.8%, which is lower than the prespecified performance goal of 5.5% (P=0.01).
| Device Success (Implantation of WATCHMAN FLX without in-hospital mortality) | 100% |
| Technical Success (Successful deployment and release, no conversion to TEE and effective closure of LAA at implant [no leak <5mm]) | 100% |
| Procedural Success (Device success plus absence of in-hospital device or procedure-related CEC adjudicated events) | 96%* |
Conversion to standard TEE during implant | 0.0% |
Total fluoroscopy time (minutes) | 19.7±12.4 |
| Procedure Time (minutes) (time between venous access and sheath removal) | 55.2±24.1 |
Number of Implanted/Attempted Devices | 1.0±0.1 |
Clinical Endpoints within 45 days of follow-up
- Patient died from a cardiac arrest 10 days after an uncomplicated implant procedure. Patient had prior history of cardiac arrest as well as renal failure, CAD and stroke and the death was not considered to be related to the procedure or device
- No patients experienced a stroke, systemic embolism, pericardial effusion, device-related thrombus, device migration or embolization
- Three patients experienced major bleeding (BARC Type 3a)
Summary and Conclusions
- We found the use of ICE during WATCHMAN FLX LAA Closure Device implantation was safe for the treatment of non-valvular atrial fibrillation in the subject population studied
- ICE-guided WATCHMAN FLX implantation demonstrated excellent procedural success, high effective LAA closure rates, and fewer short-term complications

Introduction to ICE LAA Trial
Disclosures
Clinicaltrials.gov NCT04196335. ICE LAA was funded by BSC. Disclosures- JEN-K: Principal Investigator; DA: Boston Scientific, Abbott, SMT and Ivascular. TC, DJA: full-time employees and stockholders in BSC. All other authors have no disclosures to declare. The authors thank Kristine Roy, PhD for writing/editing assistance and Wen Hsieh, PhD (paid employees of BSC) for assistance with statistical analysis.

INSIDE SCOOP:
Discussion of the PINNACLE FLX Clinical Trial
Dr. Ian Meredith, Executive Vice President, Global Chief Medical Officer, Boston Scientific