DC Bead LUMI™
Radiopaque Microsphere
DC Bead LUMI is the world’s first microsphere offering inherent, long-term radiopacity combined with the trusted performance of DC Bead
Product Description
DC Bead LUMI™ — See More. Treat Smarter
VISION
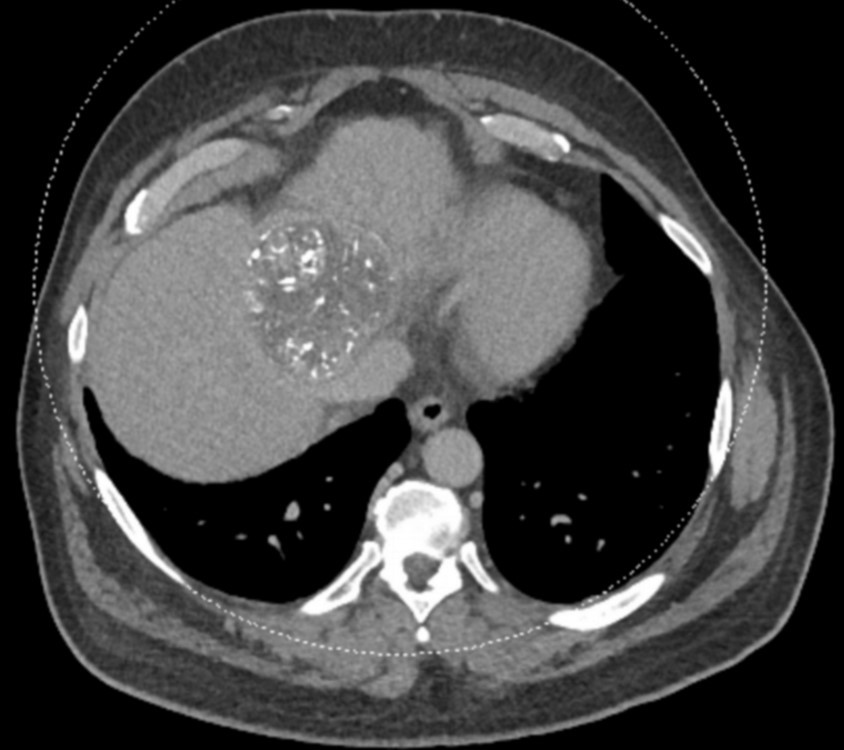
With DC Bead LUMI™, you are empowered to see the bead during and after treatment.
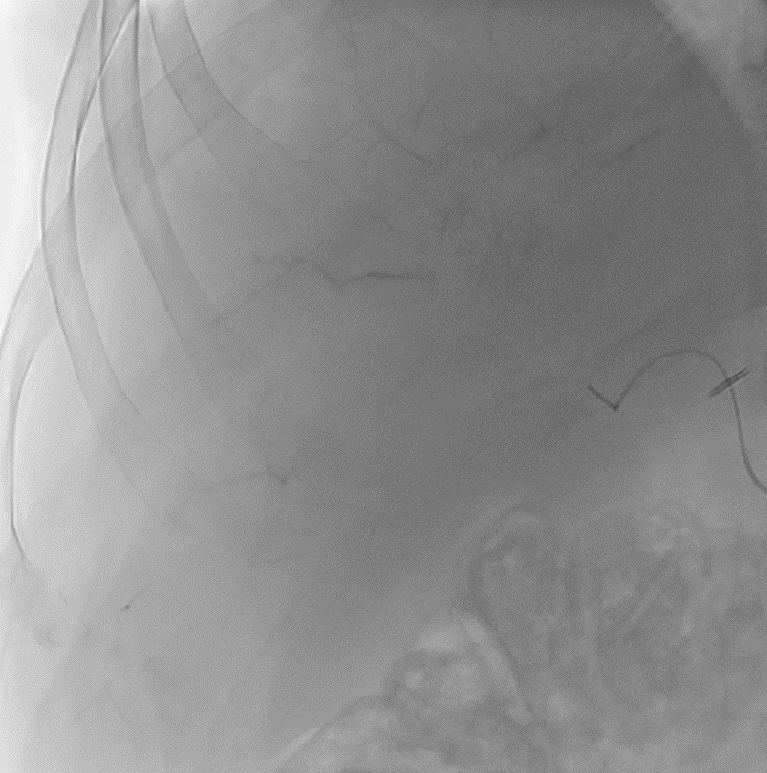
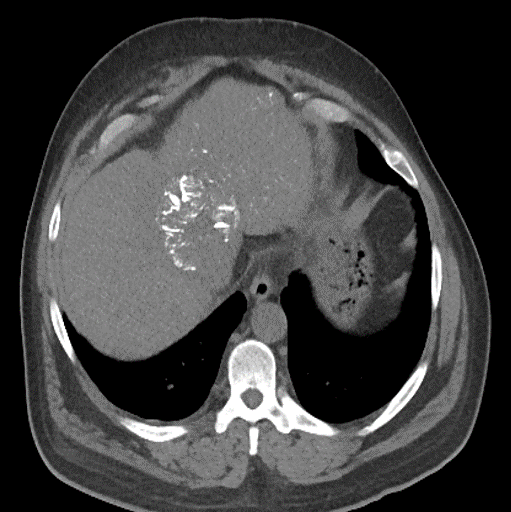
During LUMI embolization — see precisely where DC Bead LUMI™ need to be delivered1.
Fluoroscopy Single shot; images courtesy NIH with permission*.
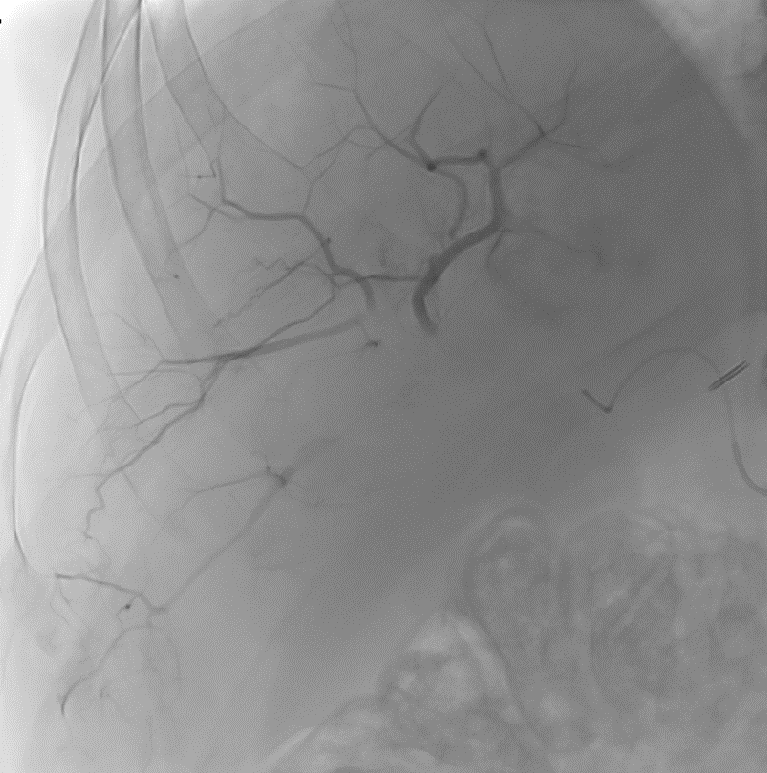
After LUMI embolization — with lasting radiopacity, DC Bead LUMI™ continues to be visible in follow-up scans1
ACC: adenoid cystic carcinoma; CBCT MIP: Cone-beam computed tomography maximum intensity projection; DSA: digital subtraction angiography; HCC: hepatocellular carcinoma; MDCT: multidetector computed tomography; NET: neuroendocrine tumor. Images courtesy of NIH with permission*.
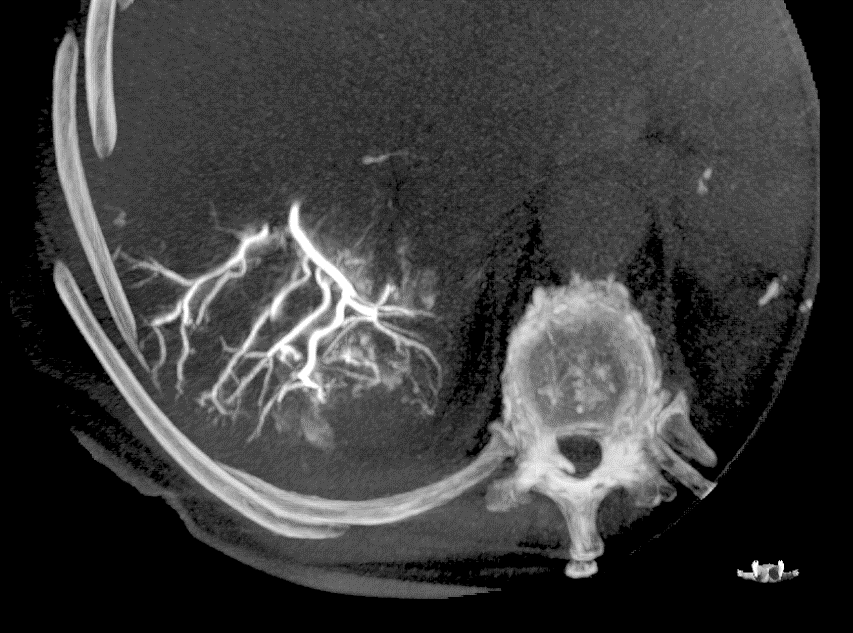
DC Bead LUMI™ offers you a new level of control with real-time feedback both during and after embolisation.
PRECISION
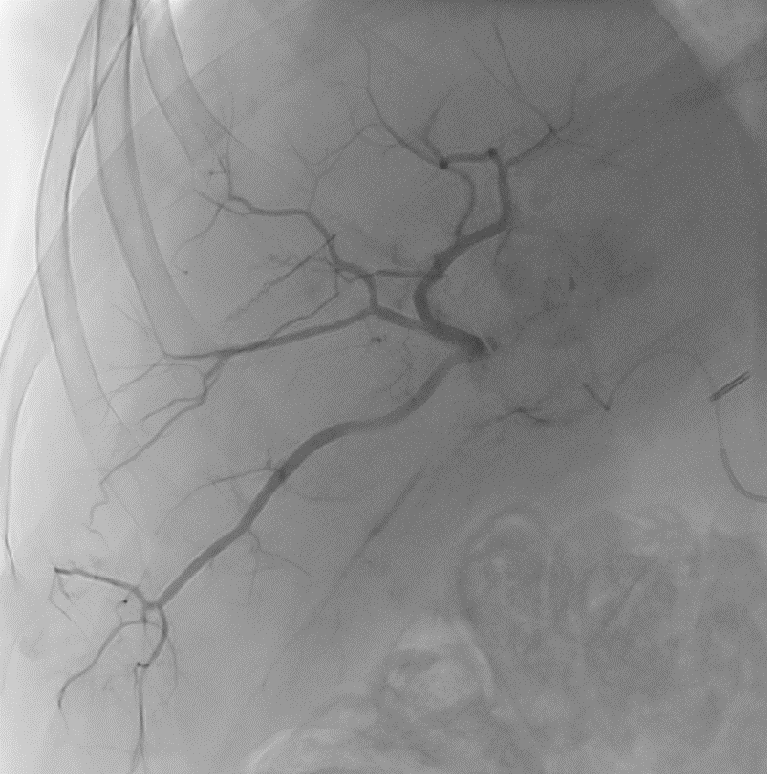
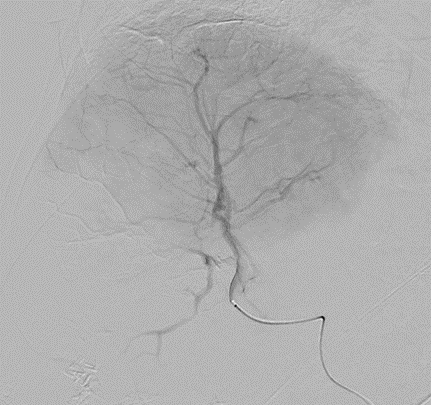
DC Bead LUMI™ is designed to offer a more precise and controlled procedure than current techniques.
CBCT MIP: Cone-beam computed tomography maximum intensity projection; DSA: digital subtraction angiography; HCC: hepatocellular carcinoma. Images courtesy of NIH with permission*.
DC Bead LUMI™ enables real-time adjustment during the whole procedure1.
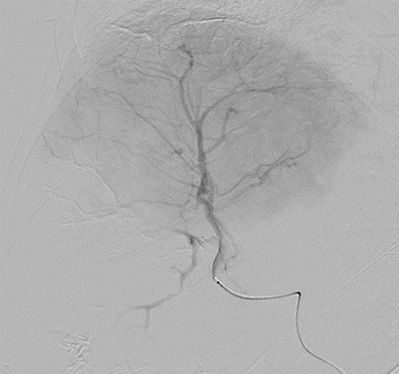
ASSURANCE
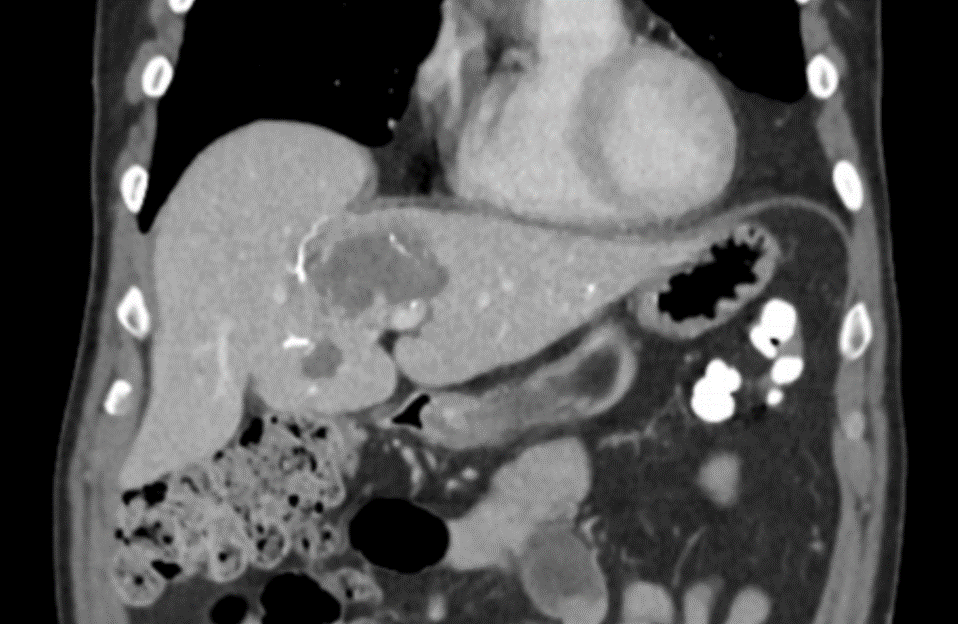
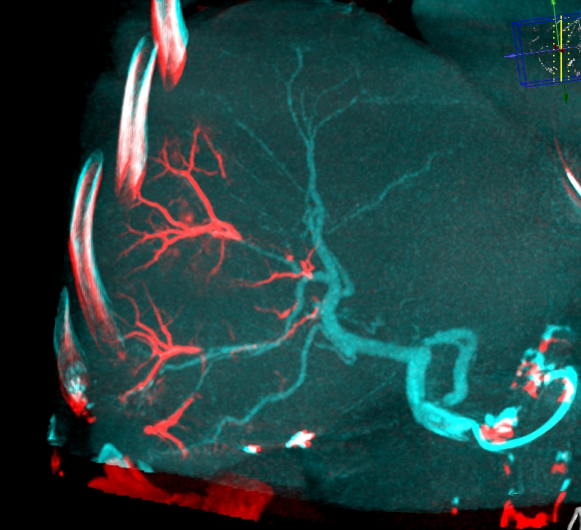
DC Bead LUMI™ offers the best opportunity to individualize your patients’ treatment.
DC Bead LUMI™ offers the precision and control you need to:
CBCT MIP: Cone-beam computed tomography maximum intensity projection; DSA: digital subtraction angiography; HCC: hepatocellular carcinoma; MDCT: multidetector computed tomography; NET: neuroendocrine tumor. Images courtesy of NIH with permission*.
Product Specifications and Sizes
DC Bead LUMI™ are precisely calibrated, radiopaque, biocompatible, nonresorbable hydrogel beads. The beads are produced from polyvinyl alcohol and contain a covalently bound radiopaque moiety.
DC Bead LUMI™ are manufactured to be inherently radiopaque and visible under imaging (Computed Tomography [CT], Cone Beam Computed Tomography [CBCT] and Fluoroscopy). DC Bead LUMI™ are available in two size ranges:
| Size | Label Color |
| 70-150µm | Black |
Presentation
- DC Bead LUMI™ are offered in a prefilled 10ml glass vial, stopper sealed by an aluminum cap with a color-coded lid.
- Each vial contains approximately 2ml of product in sterile phosphate buffered saline. The total volume of DC Bead LUMI™ and sterile physiological saline is approximately 8ml.
- Each package contains a sterile 20mm ViaLok™ Vented Vial Access Device for removal of DC Bead LUMI™ from the vial.
- Each vial of DC Bead LUMI™ is intended for single patient use only. Do not re-sterilize. Discard any unused material.
| Attribute | DC Bead LUMI |
| Radiopacity | Developed from DC Bead chemistry with a covalently bonded iodine to offer long term radiopacity. |
| Color | Pale golden color |
| Density | Density - 1.30; Iodine content makes DC Bead LUMI a little denser than DC Bead |
| Suspention | Due to DC Bead LUMI being denser, it shoudl be delivered in pure contrast to endure a good suspension. |
| Distal Penetration | In-vitro testing of DC Bead LUMI indicates that the distal penetration is equivalen to sizes of DC Bead. |
| Storage & Shelf Life | Unopened: 24 month shelf life if stored at room temperature. Opened: Once opened, should be used within 24 hours if refrigerated or 4 hours if at room temperature. |
| Packaging | New packaging containing vials with colored stoppers, transfer device. Each vial contains 2ml of beads |
Contrast Agents
Recommended Catheters and Contrast Agents for application with DC Bead LUMI
| DC Bead LUMI | Recommended Catheter (Internal Diameter) |
| 70-150μm | ≥2.0Fr (0.483mm/0.019in) |
- Other contrast agents have not been tested in conjunction with DC Bead LUMI.
- Isovue 300 (Iopamidol) has been tested and is not recommended for use due to the inadequate suspension times. Contrast agents of a similar or lower viscosity at 20° C should not be used with DC Bead LUMI.
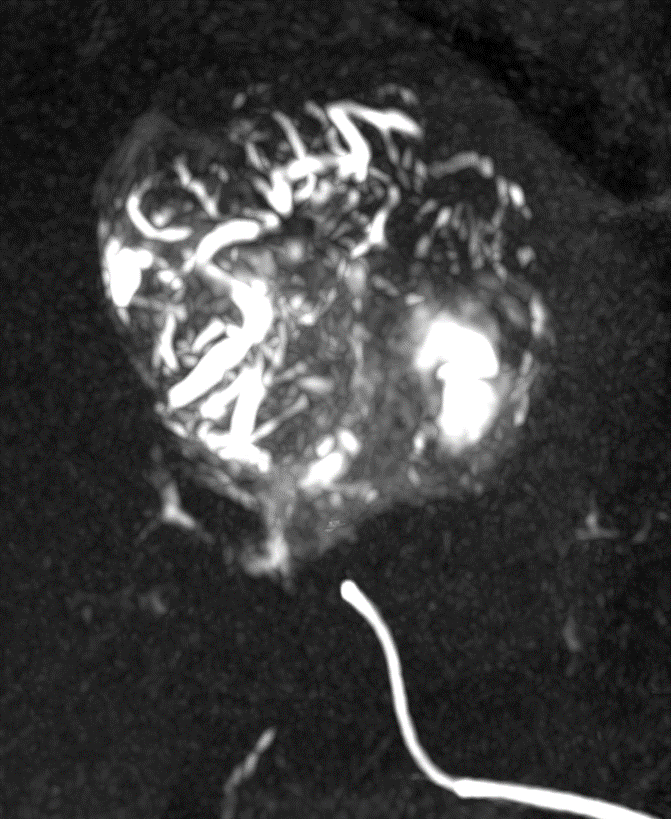
Radiopacity and Visualization
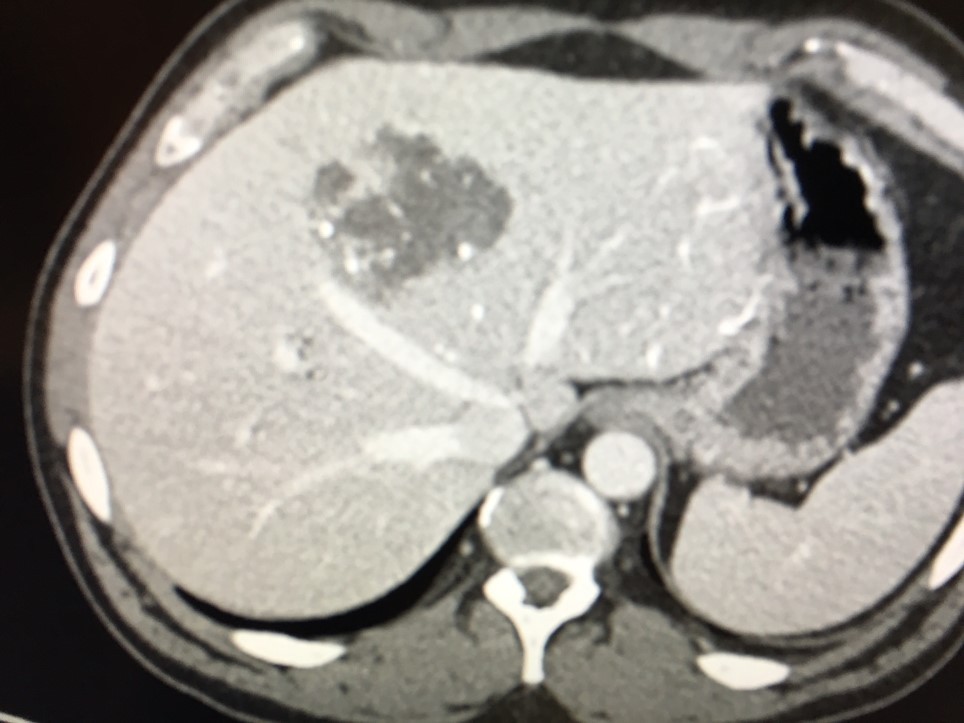
DC Bead LUMI is visible under x-ray imaging as they accumulate in the embolized vessels. It can be directly visualized as the delivery contrast agent washes out. Best visualization is achieved after administration using X-ray single shot technique (also referred to as X-ray snap shot). Cone beam CT (CBCT) can also be used to visualize the beads accumulated in the vessels with multi-planar reconstructions providing 3D spatial bead location and vessel connectivity.
DC Bead LUMI is easily visualized with CT. Early experience images from various different tumor types, show discrete embolized vessels where DC Bead LUMI is present with no significant streak artefacts nor masking of adjacent tissue unlike commonly seen following lipiodol containing treatments. If contrast enhanced CT images are desired, obtaining a non-contrast image may be helpful to discriminate DC Bead LUMI from contrast enhancement.
The radiopacity of DC Bead LUMI does not degrade over time, so the beads will continue to be visible at follow up.
Drug Loading and Elution
- Approved for loading with doxorubicin and irinotecan.
- In-vitro testing confirms that the controlled and sustained elution profiles of drug from DC Bead LUMI™are comparable to those achieved with DC Bead™
- Elution of doxorubicin and irinotecan falls within the same window as DC Bead™1
- Under the same conditions, both DC Bead LUMITM and DC BeadTM achieve complete elution of doxorubicin and irinotecan 1,2
- DC Bead LUMITM do not reduce in diameter when loaded with drug.
2 Data on file (FAR-ES-0121) at Biocompatibles UK