EMBLEM™ S-ICD System
Subcutaneous Implantable Defibrillator
Products shown for informational purposes only. Not meant as a promotion or offer for sale. Certain components are pending CE Mark. Not available for sale in the European Economic Area (EEA).
Why Modular CRM Therapy?
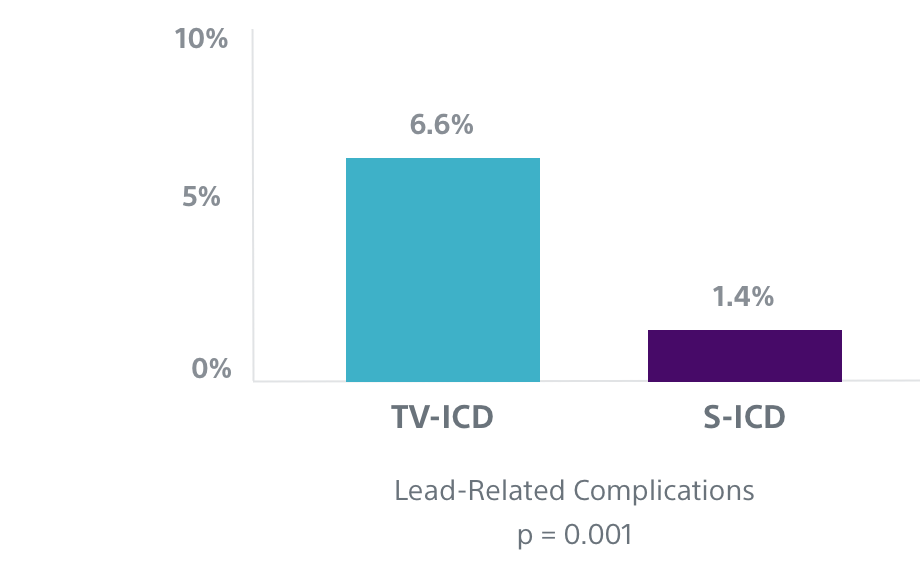
Fewer Lead-Related Complications Seen in the PRAETORIAN Trial3
The EMBLEM S-ICD leaves the vasculature untouched, thereby reducing the risk of acute and future complications associated with transvenous leads.
In the first prospective randomized head-to-head trial comparing the performance of S-ICD and TV-ICD, data showed that TV-ICD patients experienced more than four times as many lead-related complications as S-ICD patients.3
EMPOWER™ Leadless Pacemaker Procedure Videos
How mCRM Therapy Works
1. When the EMBLEM S-ICD senses an episode of tachycardia, it triggers the EMPOWER Leadless Pacemaker to provide ATP therapy, which may stop the tachycardia and reset the heart to a normal rhythm.
2. The EMBLEM S-ICD continuously monitors for the result of the ATP therapy.
a. If the ATP successfully terminated the tachycardia and returned the heart to a normal rhythm, the EMBLEM S-ICD will continue monitoring and no shock will be delivered.
b. If the ATP did not terminate the tachycardia, the EMBLEM S-ICD will deliver shock therapy.
Example of mCRM Therapy
(Tjong, F.V.Y., et al. HRS 2016)
Watch the video to see the coordinated therapy between the EMBLEM S-ICD and EMPOWER Leadless Pacemaker during a mCRM therapy sequence.
mCRM System Components
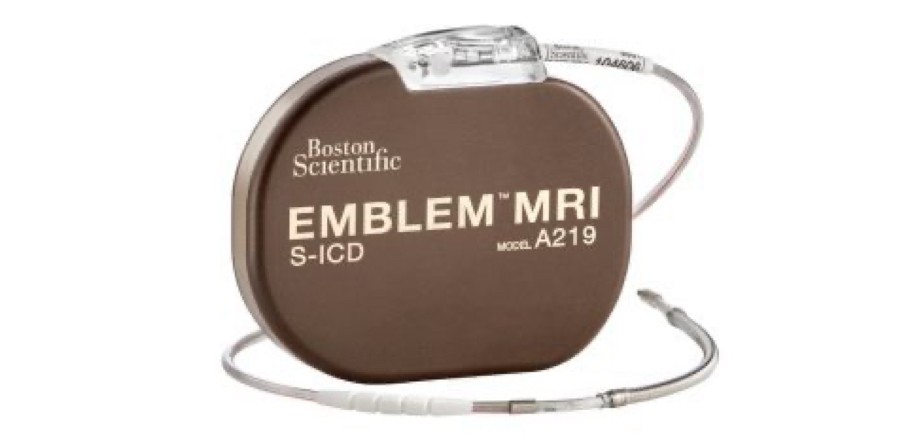
EMBLEM S-ICD
The only extrathoracic implantable defibrillator that provides protection from both sudden cardiac death and the risks and complications associated with transvenous leads.
- Eliminates potential for vascular injury, transvenous lead insertion complications, lead-associated tricuspid regurgitation, mechanically induced pro-arrhythmia, and transvenous lead failure and associated extraction risk
- Reduces risk of systemic
- Preserves the vasculature
- Remains outside the ribcage, never touching the heart

EMPOWER Leadless Pacemaker (LP)*
The EMPOWER Leadless Pacemaker is designed to be paired with S-ICD to provide pacing or ATP therapies at the time they are needed.
- ATP when commanded by a paired S-ICD
- Delivery system with inner extendable shaft
- 20.7 F delivery catheter
- Dedicated retrieval catheter
- Rate response via accelerometer
- > 10 Year average longevity expected when used primarily for ATP therapy as part of an mCRM System
Are You a Clinical Site Investigator?
MODULAR ATP Study
Learn about the MODULAR ATP Clinical Study design, primary effectiveness and safety endpoints, timelines and more.