Catheters: Ablation
All Specialties (10)
-
BLAZER PRIME™ Temperature Ablation Catheter
Intuitively engineered construction combined with our trusted BLAZER™ Catheter platform provides an ablation catheter designed to function as a physical extension of your hand. Manipulation of the handle translates predictably into movement of the catheter tip. Control and durability provide procedural confidence.
- Electrophysiology
-
BLAZER™ II Temperature Ablation Catheter Family
Exceptional Clinical Results
- Electrophysiology
-
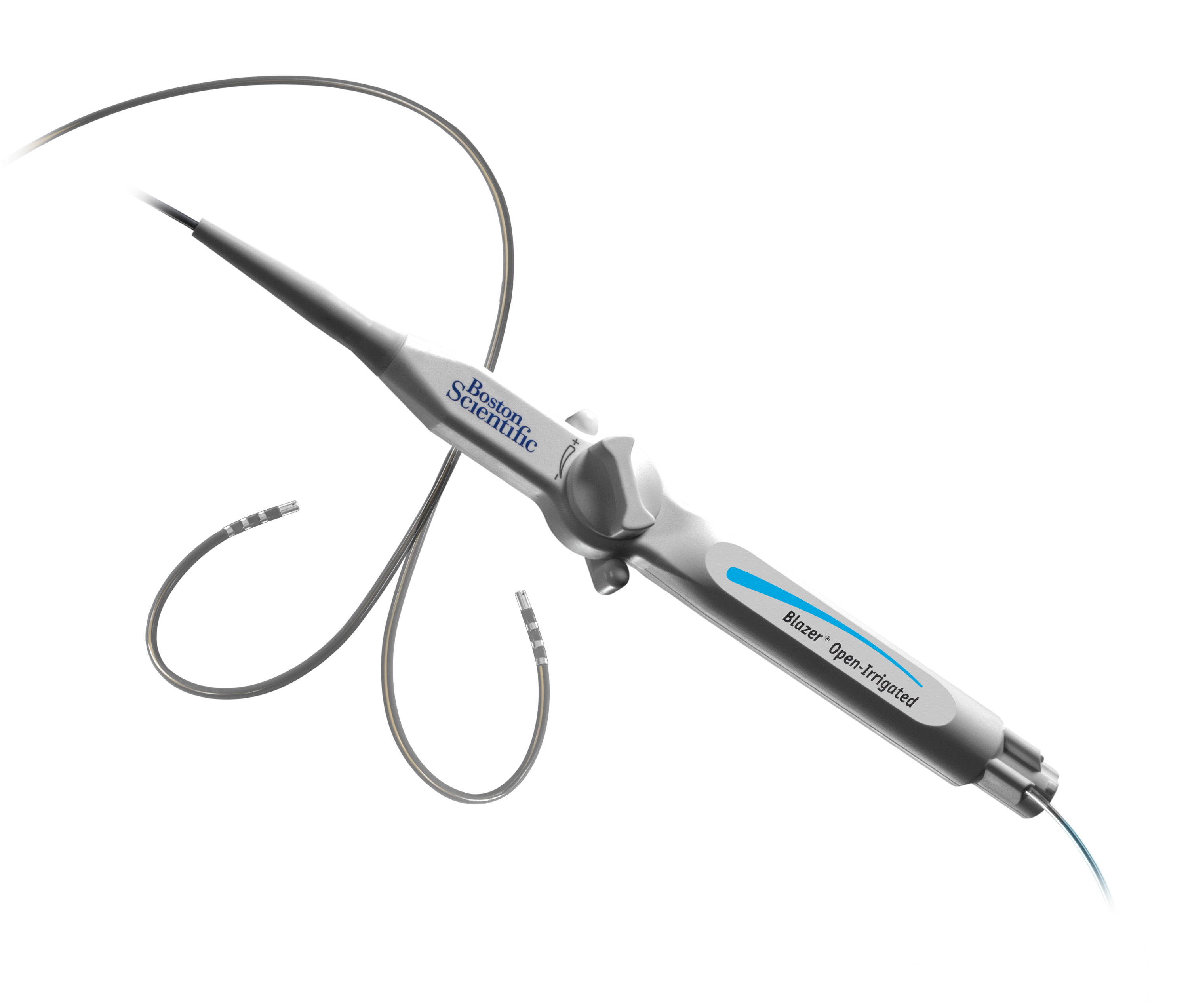
Blazer™ Open-Irrigated Ablation Catheter
The Blazer™ Open-Irrigated Ablation Catheter is the only catheter with a Total Tip Cooling™ Design which provides total uniform tip cooling internally, an optimized flow pattern with active washing and consistent cooling throughout RF delivery as well as a uniform external flow.
- Electrophysiology
-
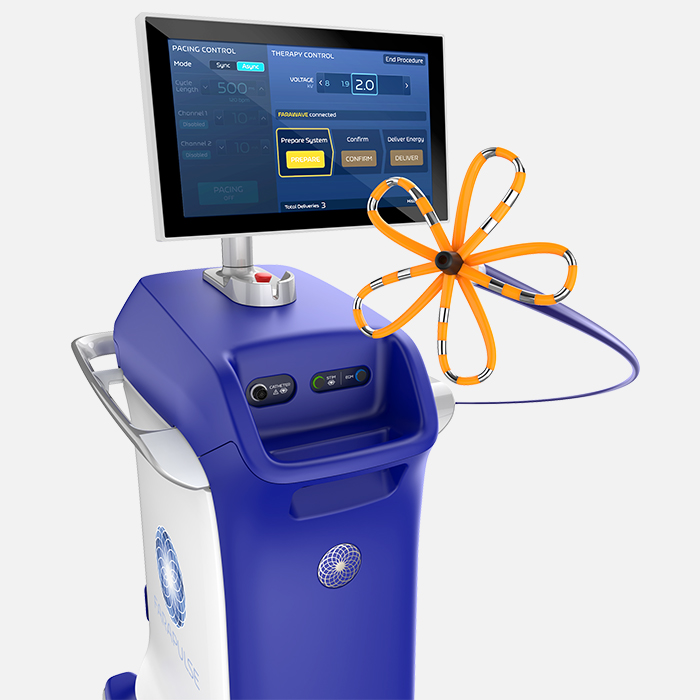
FARAPULSE™ Pulsed Field Ablation System
The most-proven PFA system transforming treatment for Afib
FARAPULSE Pulsed Field Ablation (PFA) is a non-thermal mechanism of ablation, delivering durable and effective lesions while reducing many traditional risks associated with thermal ablation. It has been used to treat over 200,000 patients, positively transforming patient outcomes and clinical practice.- Electrophysiology
-
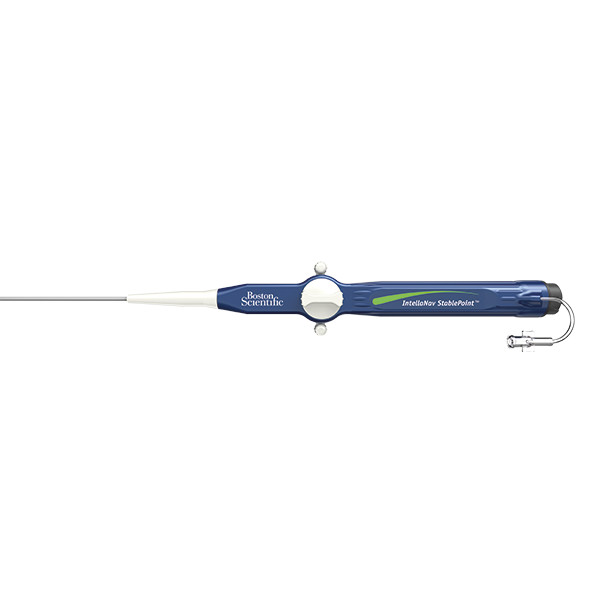
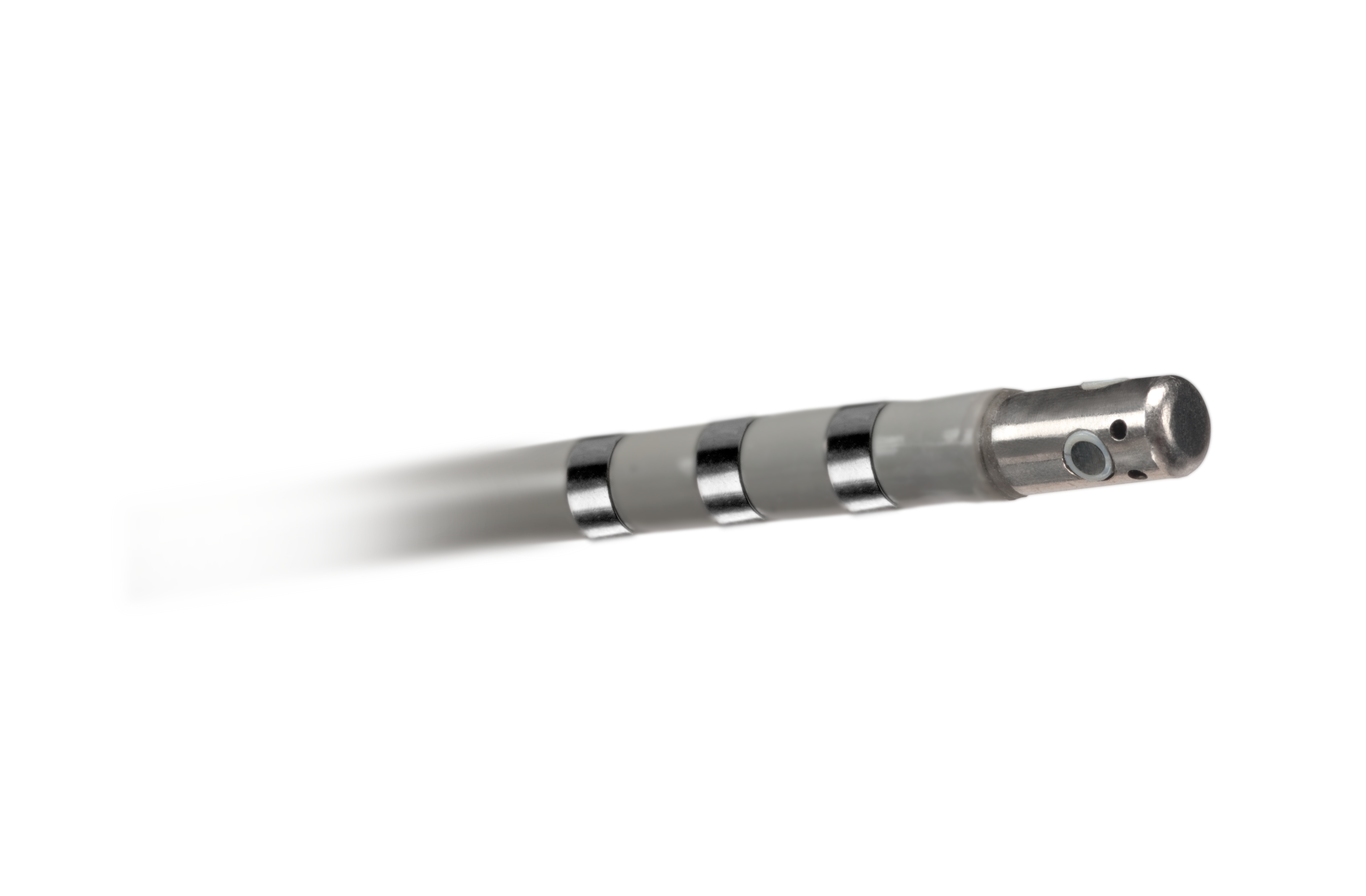
INTELLANAV STABLEPOINT™ Ablation Catheter
INTELLANAV STABLEPOINT™ Ablation Catheter, enabled with DIRECTSENSE™ Technology, combines the power of contact force and local impedance to give you dynamic insights at and below the cardiac tissue surface. Unlike any other technology, this integrated solution, available exclusively via OPAL HDx™, enables you to diagnose, ablate, and validate with more critical information than ever before.
- Electrophysiology
-
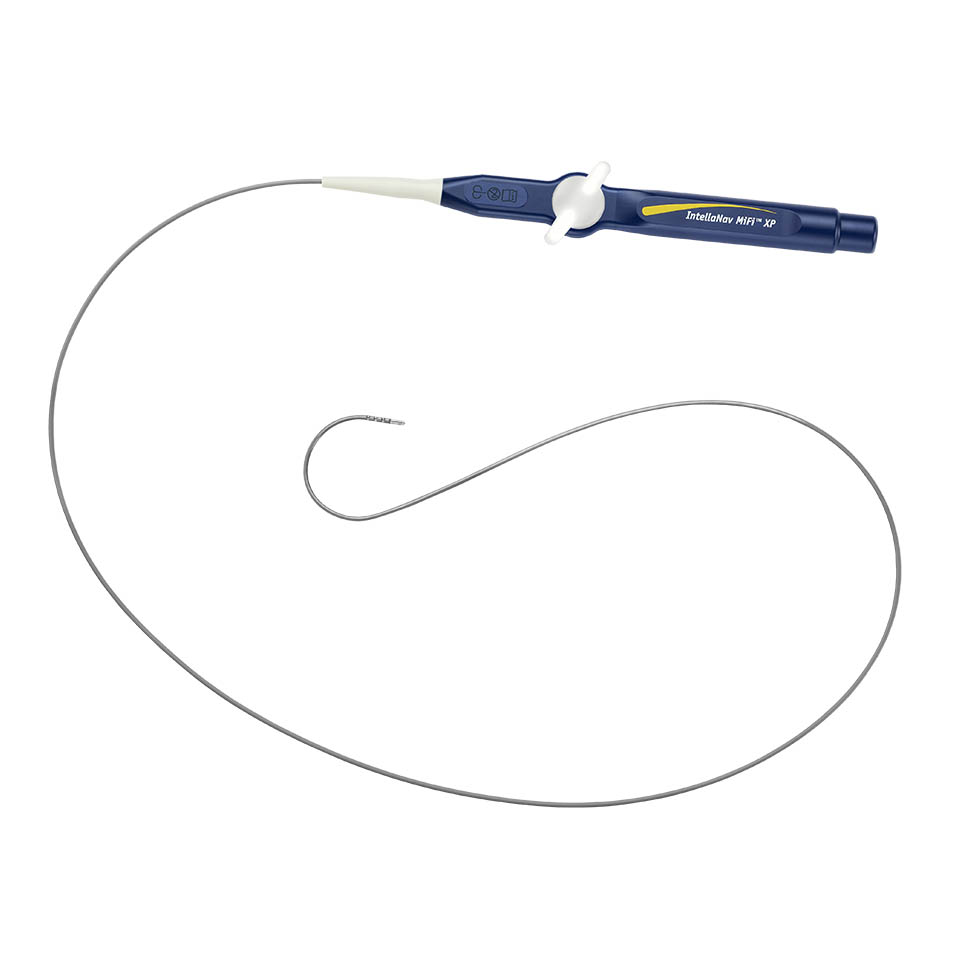
INTELLANAV MIFI™ XP Temperature Ablation Catheter Family
The INTELLANAV MIFI™ XP catheters encompass the proven performance of the BLAZER™ XP platform and the unparalleled clarity of MIFI technology in our magnetically tracked ablation catheters. These catheters unlock the magnetic tracking performance of the RHYTHMIA™ Mapping System, enabling increased accuracy, clarity, and performance.1
- Electrophysiology
-
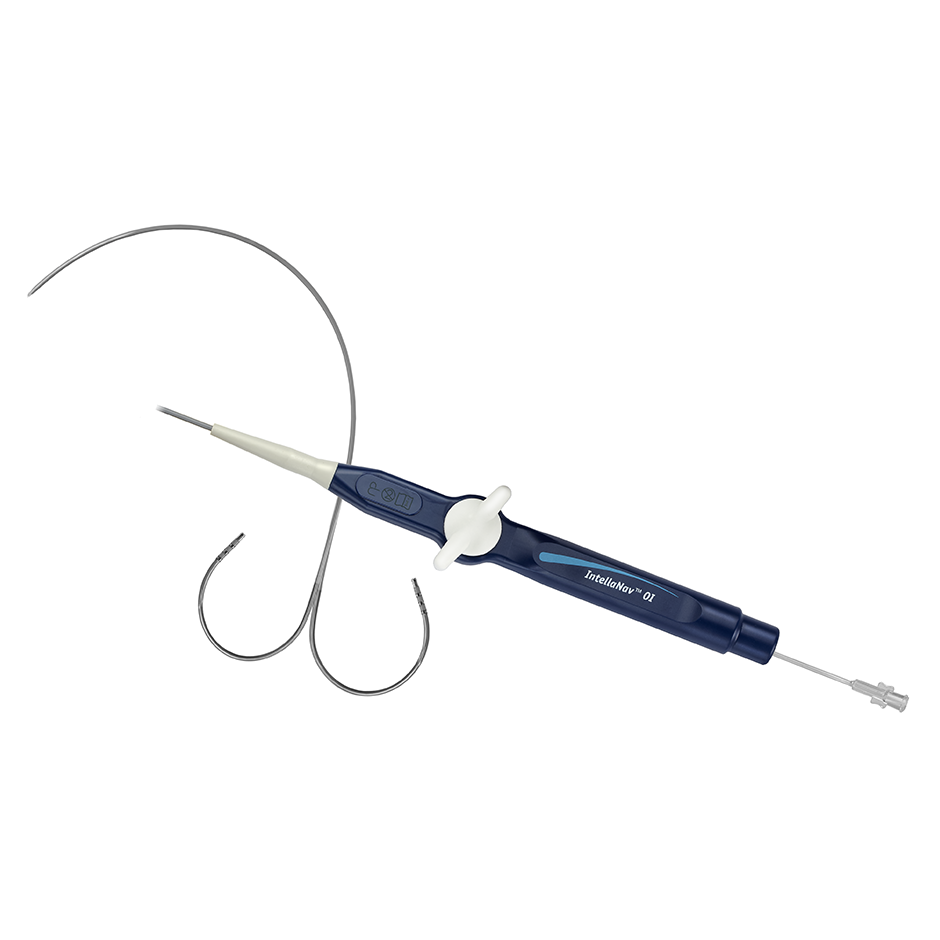
INTELLANAV™ MIFI OPEN-IRRIGATED Ablation Catheter
The INTELLANAV MIFI Open Irrigated Catheter brings together our proprietary Mini-Electrode technology with the Total Tip Cooling design and the IntellaNav magnetic tracking for Unparalleled Clarity, Cool Performance and Confident Navigation in Rhythmia HDx Mapping System procedures
- Electrophysiology
-
INTELLANAV™ OPEN-IRRIGATED Ablation Catheter
The INTELLANAV™ OPEN-IRRIGATED catheter encompasses the elegant Total Tip Cooling™ design and the familiarity and proven performance of the Blazer platform, now enhanced with INTELLANAV magnetic tracking technology for cool performance and confident navigation in RHYTHMIA Mapping System procedures.
- Electrophysiology
-
INTELLATIP MIFI™ Open-Irrigated (OI) Multi-Dimensional Ablation Catheter
The INTELLATIP MIFI™ OI Ablation Catheter delivers the critical, multi-dimensional information you need to confidently diagnose and treat complex cardiac arrhythmias and provide the true picture of exactly what is happening at the tip of the ablation catheter in real-time.
- Electrophysiology
-
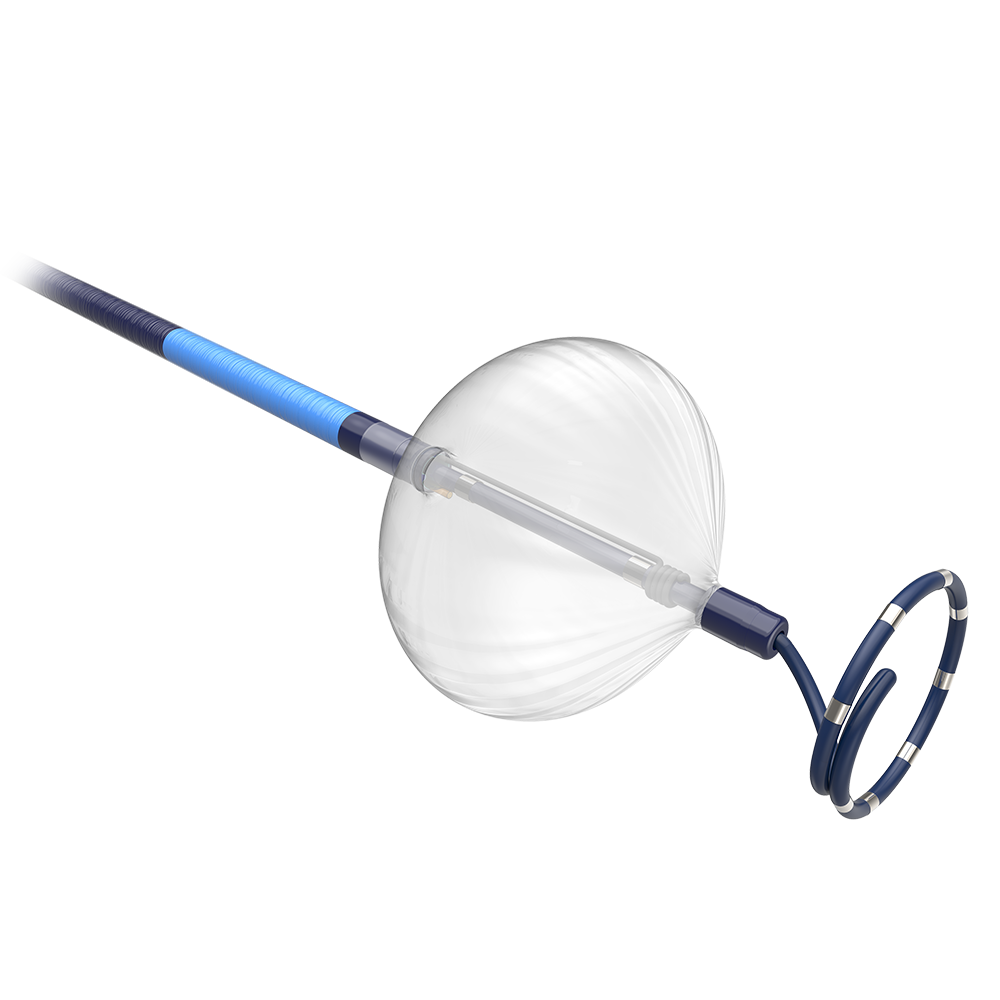
POLARx™ Cryoablation System
The POLARx Cryoablation System has been designed to retain the best of the established approach and performance of cryoablation, then goes a step further to implement physician-driven changes to address well-documented limitations.
- Electrophysiology