Drug-Eluting Stents (DES) FAQs
Figure 1: Eluvia Drug-Eluting Vascular Stent System

Drug-Coated Balloons (DCB) FAQs
Figure 4: Ranger Paclitaxel-Coated PTA Balloon Catheter
Resources
[1] Payne MM. Charles Theodore Dotter. The father of intervention. Tex Heart Inst J 2001;28:28-38. Secemsky EA, Shen C, Schermerhorn M, et al. Longitudinal assessment of safety of femoropopliteal endovascular treatment with paclitaxel-coated devices among Medicare beneficiaries: The SAFE-PAD study. JAMA Intern Med 2021;181:1071-80.
[2] Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vasc Med 2017;22:NP1-N43.
[3] Ibid.
[4] ELEGANCE Registry https://clinicaltrials.gov/ct2/show/NCT04674969
[5] Gray WA, Keirse K, Soga Y, Benko A, et al; IMPERIAL investigators. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet 2018;392:1541-51.
[6] Ibid.
[7] McKeown, LA. EMINENT: Better Patency With DES Over BMS for Fem-Pop Lesions. TCTMD Oct 2021. Available at https://www.tctmd.com/news/eminent-better-patency-des-over-bms-fem-pop-lesions. Last accessed 25/05/2022.
[8] In EMINENT, primary sustained clinical improvement was defined as an improvement (decrease) by at least 1 Rutherford category, without TLR.
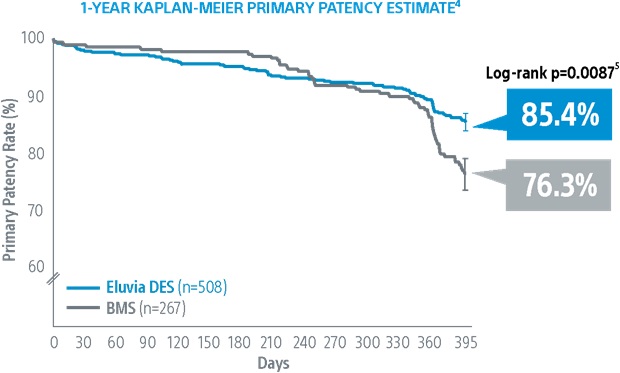
[9] Kaplan-Meier Estimate: Primary patency defined as core-lab assessed duplex ultrasound peak systolic velocity ratio (PSVR) ≤ 2.4 at 1-year in the absence of clinically-driven TLR or bypass of the target lesion.
[10] Gray et al., IMPERIAL investigators.
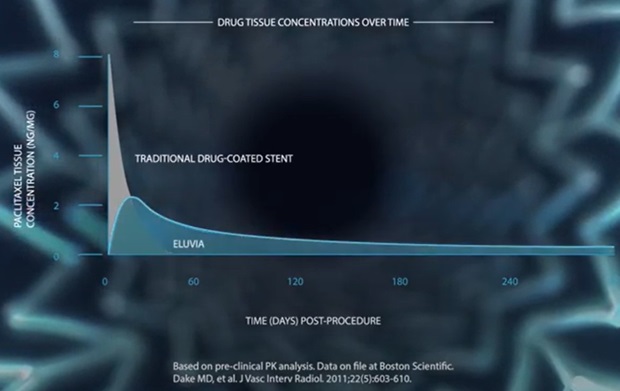
[11] Speck U, Stolzenburg N, Peters D, et al. How does a drug-coated balloon work? Overview of coating techniques and their impact. J Cardiovasc Surg (Torino) 2016;57:3-11.
[12] Steiner S, Schmidt A, Zeller T, et al. COMPARE: prospective, randomized, non-inferiority trial of high vs. low-dose paclitaxel drug-coated balloons for femoropopliteal interventions. Eur Heart J 2020;41:2541-52.
[13] Ibid.
[14] RANGER™ Paclitaxel Coated Balloon vs Standard Balloon Angioplasty (RANGER II SFA) Study Record Detail, https://clinicaltrials.gov/ct2/show/NCT03064126. Last accessed 25/05/2022.
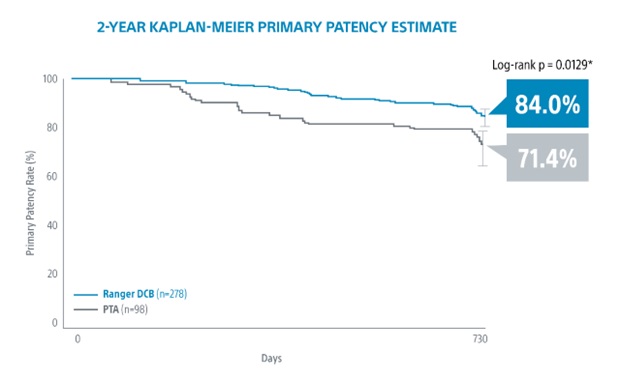
[15] RANGER II SFA RCT 2-Year Results presented by Ravish Sachar, MD. VIVA 2021.
[16] Ibid.
[17] Secemsky EA, Shen C, Schermerhorn M, et al. Longitudinal assessment of safety of femoropopliteal endovascular treatment with paclitaxel-coated devices among Medicare beneficiaries: The SAFE-PAD study. JAMA Intern Med 2021;181:1071-80.
[18] Katsanos K, Spilopoulos S, Kitrou P, et al. Risk of Death Following Application of Paclitaxel‐Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials. Journal of the American Heart Association. 2018;7:e011245.
[19] Bonaca M, Bauersachs R, Anand S, et al. Rivaroxaban in Peripheral Artery Disease After Revascularization. N Engl J Med 2020; 382:1994-2004.
[20] Dinh K, Limmer A, Paravastu S, et al. J of Endovascular Therapy, Volume: 26 issue: 5, page(s): 600-612.
[21] Nordanstig J, James S, Andersson M, et al. Mortality with Paclitaxel-Coated Devices in Peripheral Artery Disease. N Engl J Med 2020; 383:2538-2546.
[22] Secemsky EA, et al.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.