Five-Year Data
Results of a five-year clinical trial confirming long-term durability of the Rezūm Water Vapour Therapy for treatment of benign prostatic hyperplasia (BPH) were published online in the Journal of Urology. The study found Rezūm provided significant, sustained improvement of lower urinary tract symptoms (LUTS) and quality of life for patients suffering from BPH out to five years post-procedure.
Study Design
Patient Profile
197 subjects ≥ 50 years old
International Prostate Symptom Score (IPSS) | Maximum flow rate (Qmax) | Prostate volume |
| ≥13 | 5-15 ml/s | 30-80 cc |
Outcomes Measured
- Changes in:
- International Prostate Symptom Score (IPSS)
- Quality of life (QOL)
- Maximum flow rate (Qmax)
- BPHII
- Retreatment of BPH after the index procedure:
- Secondary surgical treatment for LUTS/BPH
- Initiated BPH medication (alpha-blocker, or 5-ARIs)
Results
Sustains Clinical Outcomes through Five Years1

Rezum Water Vapour Therapy
Provides durable significant improvement for BPH patients.
Sustained symptom relief and QOL improvement
Compared to baseline
Versatile to Treat Median Lobe Obstruction(MLO)

- Rezūm can treat lateral and central zones without physicians having to learn an advanced technique
Positive Safety Profile and Preserves Sexual Function
- No late occuring related adverse events or de novo erectile dysfunction reported at 5 years
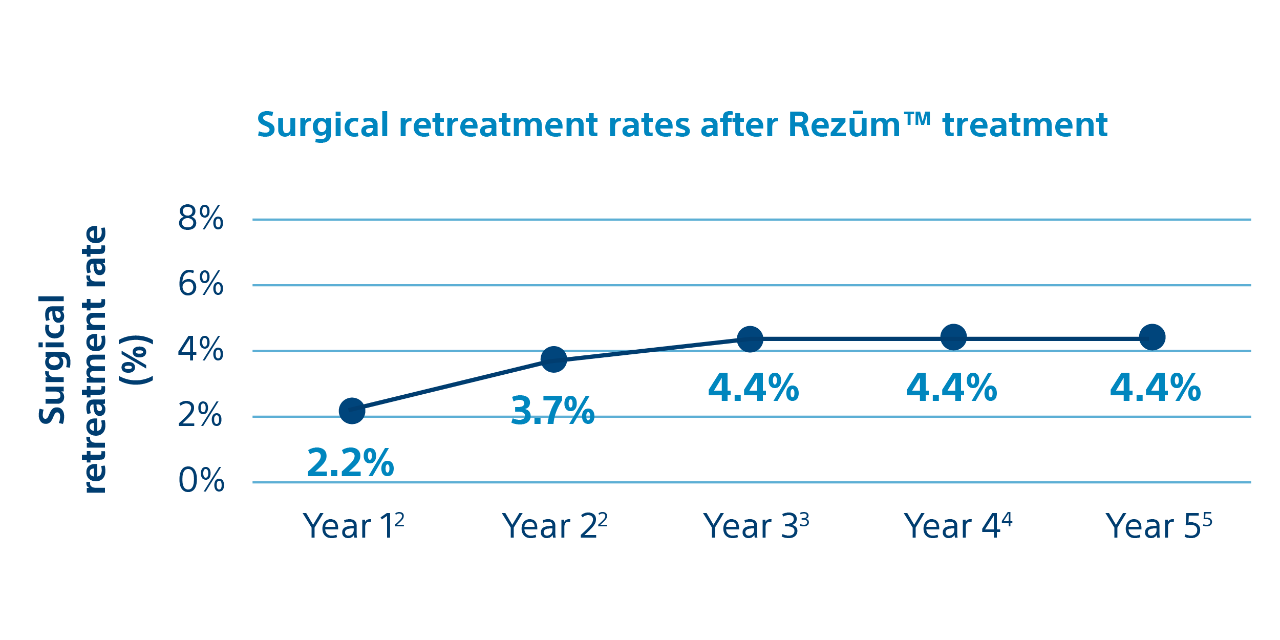
Rezūm II Clinical Trial Retreatment Rates through Five Years1,6
"These new five-year results expand upon the growing clinical evidence supporting the long-term durability of the Rezūm System as the leading minimally invasive option for men facing moderate-to-severe BPH-related symptoms."
Kevin McVary, MD, FACS
Director, Center for Male Health, and Professor of Urology at Stritch School of Medicine, Loyola University Medical Center and principal investigator of the Rezūm II clinical trial
About Rezūm Water Vapour Therapy
The Rezūm System addresses BPH symptoms directly by reducing adenoma tissue with water vapor through a minimally invasive, in-office procedure, allowing cell remnants to be absorbed directly by the body. The procedure is typically performed during a single visit to the physician's office and does not require general anesthesia or surgical implants. Rezūm is included in the American Urological Association's BPH treatment guidelines, with a positive safety profile through five years supporting its continued use for the reduction of prostate tissue associated with BPH.
For information purposes only. The content of these articles/publications is under the sole responsibility of its author/publisher and does not represent the opinion of BSC.
References
- McVary KT, Roehrborn C. Five year results of the prospective, randomized controlled trial of water vapor thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Late-Breaking Abstract. J Urol. 2020 Apr;203(4):e1021.
- Rezum II Trial – BSC data on file.
- McVary KT, Roehrborn CG. Three-Year Outcomes of the Prospective, Randomized Controlled Rezūm™ System Study: Convective Radiofrequency Thermal Therapy for Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia. Urology. Elsevier Inc.; 2018;111: 1–9. doi:10.1016/j.urology.2017.10.023.
- McVary KT, Rogers T, Roehrborn CG. Rezūm™ Water Vapor Thermal Therapy for Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: 4-Year Results From Randomized Controlled Study. Urology. Elsevier Inc.; 2019;126: 171–179. doi:10.1016/j.urology.2018.12.041.
- McVary KT, Roehrborn C. Five year results of the prospective, arndomized, controlled trial of water thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. J Urol. 2020;203: e1021
- Data on File with Boston Scientific.
All treatments have inherent and associated risks. The Rezūm System is intended to relieve symptoms, obstructions, and reduce prostate tissue associated with benign prostatic hyperplasia (BPH). It is indicated for men with a prostate volume ≥ 30 cm3. The Rezūm System is also indicated for treatment of prostate with hyperplasia of the central zone and/or a median lobe. Potential risks include but not limited to painful urination (dysuria), blood in the urine (hematuria), blood in the semen (hematospermia), decrease in ejaculatory volume, suspected urinary tract infection (UTI), and urinary frequency, retention or urgency. You should talk with your doctor about benefits and risks before moving forward with any treatment option.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
All images are the property of Boston Scientific.
All trademarks are the property of their respective owners.
IMPORTANT INFORMATION: These materials are intended to describe common clinical considerations and procedural steps for the use of referenced technologies but may not be appropriate for every patient or case. Decisions surrounding patient care depend on the physician’s professional judgment in consideration of all available information for the individual case. Boston Scientific (BSC) does not promote or encourage the use of its devices outside their approved labeling. Case studies are not necessarily representative of clinical outcomes in all cases as individual results may vary.




















