EMBLEM™ MRI S-ICD System
Subcutaneous Implantable Defibrillator

Overview
Primary endpoint
While superiority for the S-ICD was not established for the composite primary endpoint, the S-ICD demonstrated a significantly lower risk of major†- and lead-related complications compared to the TV-ICD.1
Investigators concluded that the S-ICD is a viable therapy option that should be considered for all ICD patients without an indication for pacing.1
Key considerations:
Device-related complication rates were not statistically different between S-ICD and TV-ICD (HR: 0.73, 95% CI 0.48-1.12, P = 0.15).1



Secondary endpoints


Over the median follow up period of 7.3 years, there was no difference in mortality, hospitalization for heart failure, or major adverse cardiac events between the two arms.1
†Major complications were those requiring an invasive intervention.
Device-related complications in transvenous versus subcutaneous defibrillator therapy during long-term follow-up: The PRAETORIAN-XL Trial

The PRAETORIAN Trial1, 2
The PRAETORIAN Trial is an investigator-sponsored study (ISR)* initiated, designed and led by Academic Medical Center in Amsterdam (AMC) and Reinoud E. Knops, MD, PhD. It is the first prospective randomized head-to-head trial comparing the performance of S-ICD and TV-ICD.
The trial hypothesis was that the S-ICD is non-inferior to the TV-ICD with respect to major ICD-related adverse events, including:
- Inappropriate shocks
- ICD-related complications that require intervention
- Lead-related complications
The trial enrolled 849 patients between March 2011 and January 2017 within the EU and US.

4-YEAR TRIAL RESULTS
Data from the PRAETORIAN trial confirmed that the S-ICD can be the preferred therapy choice over the TV-ICD for protection from sudden cardiac death. The S-ICD offers comparable performance for the majority of ICD-indicated patients who do not have a need for pacing, while avoiding the serious complications associated with TV-ICDs such as serious infections and lead-related complications.
Primary and secondary endpoints**
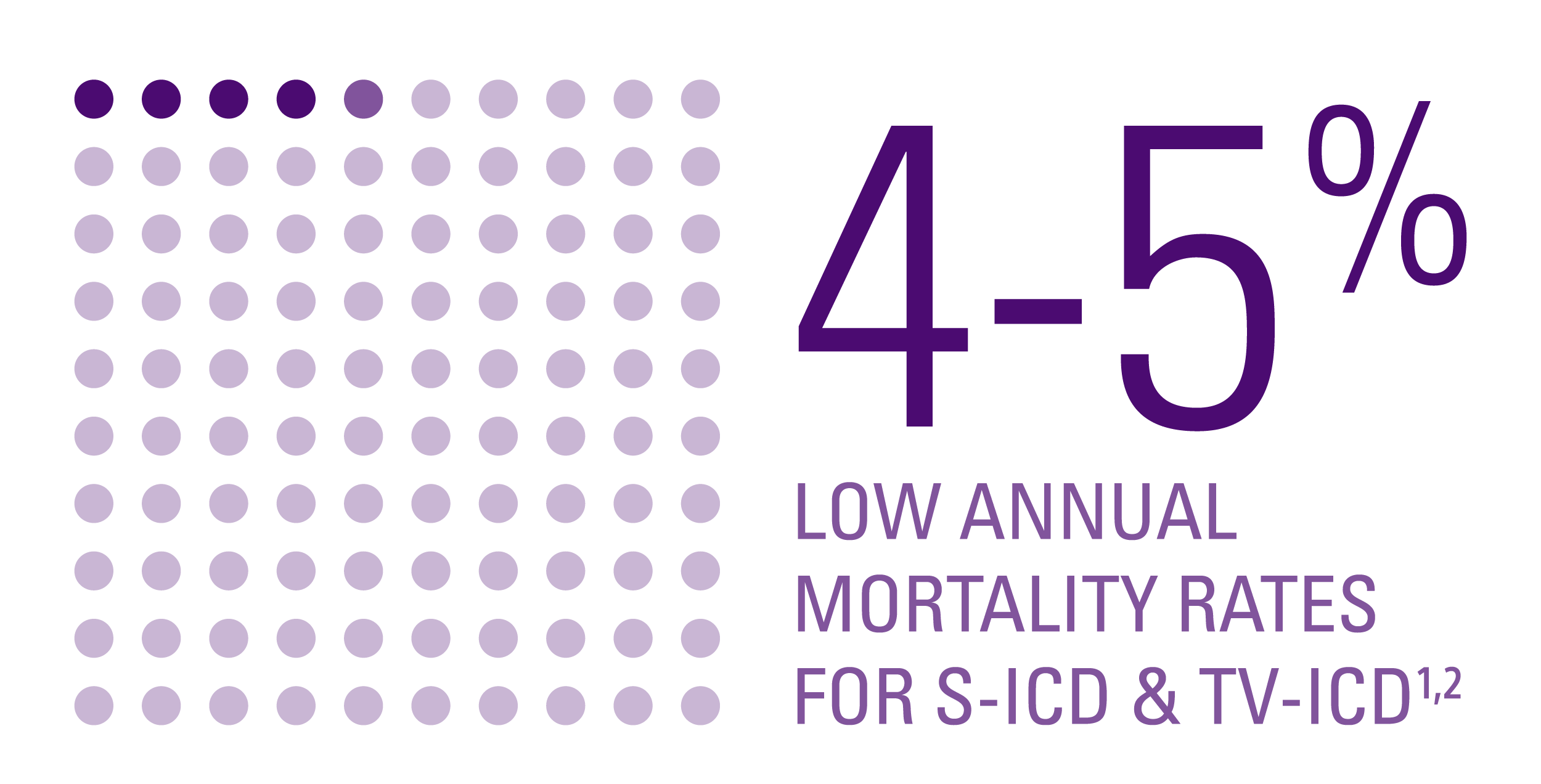
Mortality rates1,2
- No significant difference in overall and arrhythmic mortality rates between the two groups.
- Mortality rate was low in both groups, even though:
- Over 90% had ischemic (>68%) or non-ischemic heart failure
- Secondary prevention for 19% of S-ICD patients and 20% of TV-ICD patients
- Median EF was 30%
- Median age was 63 years
- Arrhythmic deaths were identical in both groups.
- Numerically, more deaths occurred in the S-ICD group; this difference was due to non-cardiac causes including cancer and gastrointestinal disease.
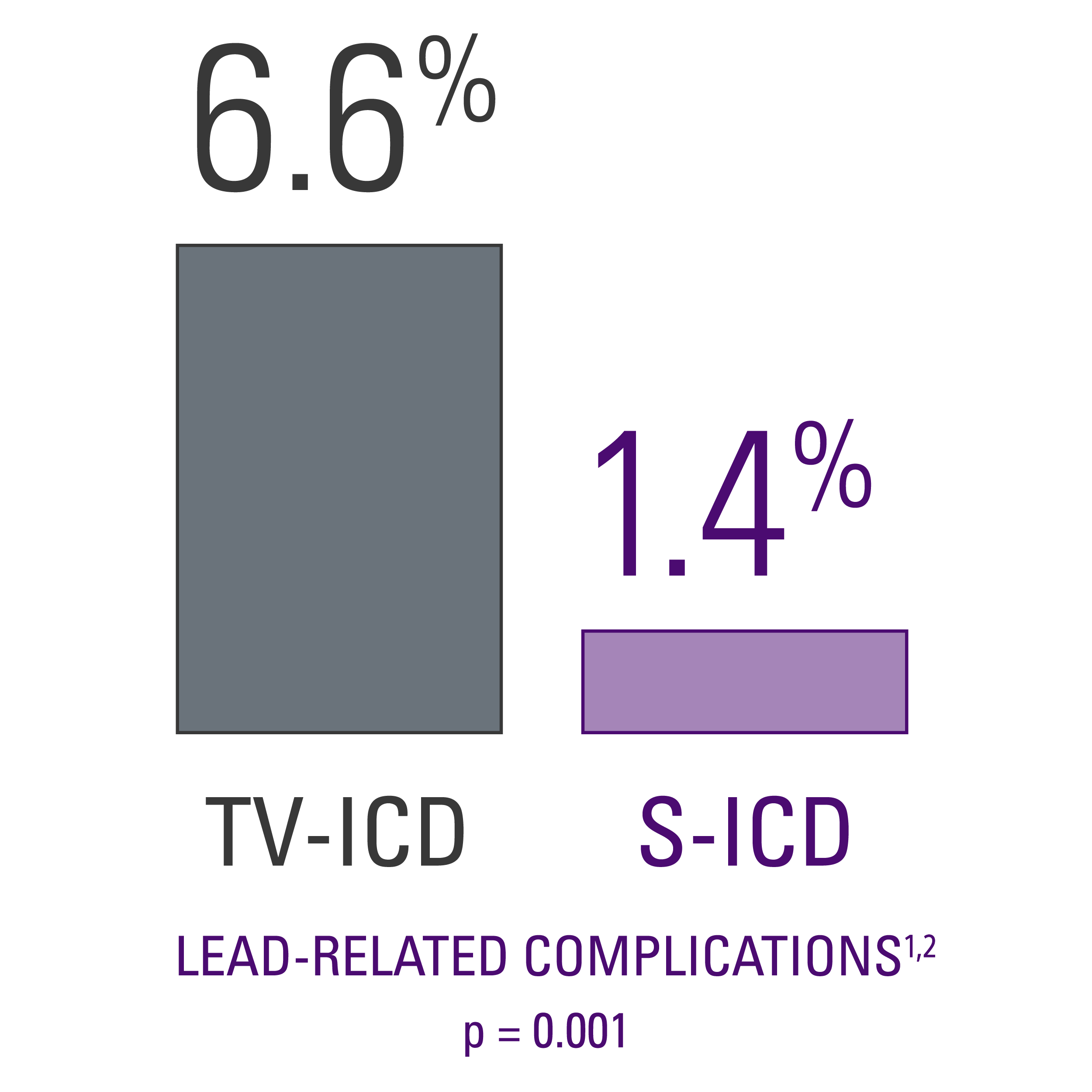
Lead-related complications
- Data showed a statistical difference in lead-related complications, with TV-ICD patients experiencing more than 4 times as many as S-ICD patients did.2
- The S-ICD leaves the vasculature untouched, thereby reducing the risk of acute and future complications associated with transvenous leads.
- Eliminating device leads within the vasculature is particularly important for ICD-indicated patients with co-morbidities such as diabetes and renal disease who often are at an increased risk of infection and may have vascular access issues.3

Infections requiring device extraction
- Numerically, TV-ICD patients experienced twice as many infections that required device extraction as S-ICD patients did.1,2
- Data in >91,000 transvenous lead extractions demonstrated that those extracted for infection had significantly higher rates of complications and mortality.4
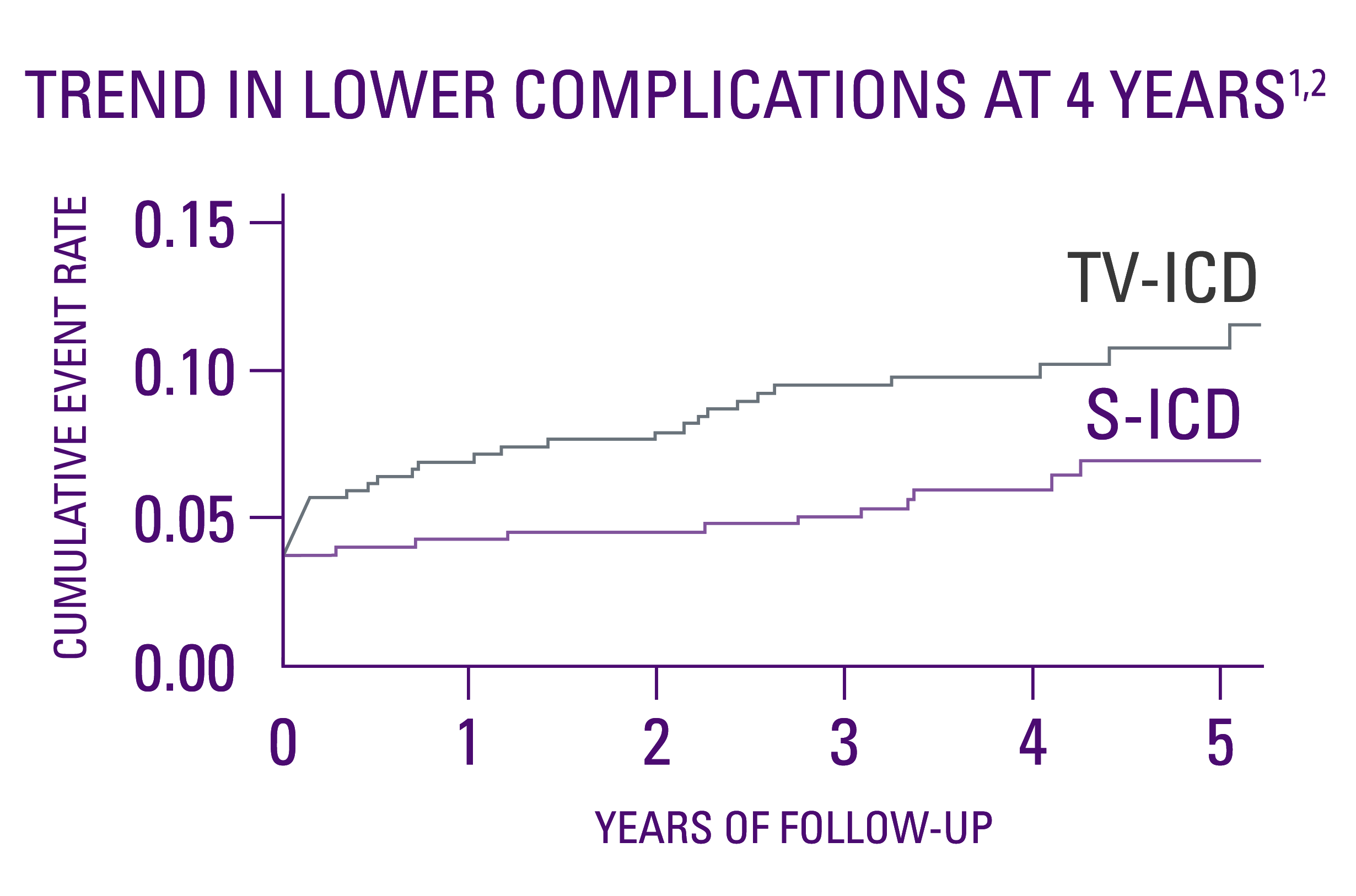
Device-related complications1,2
- No statistical difference in device-related complications at the median 4-year follow-up.
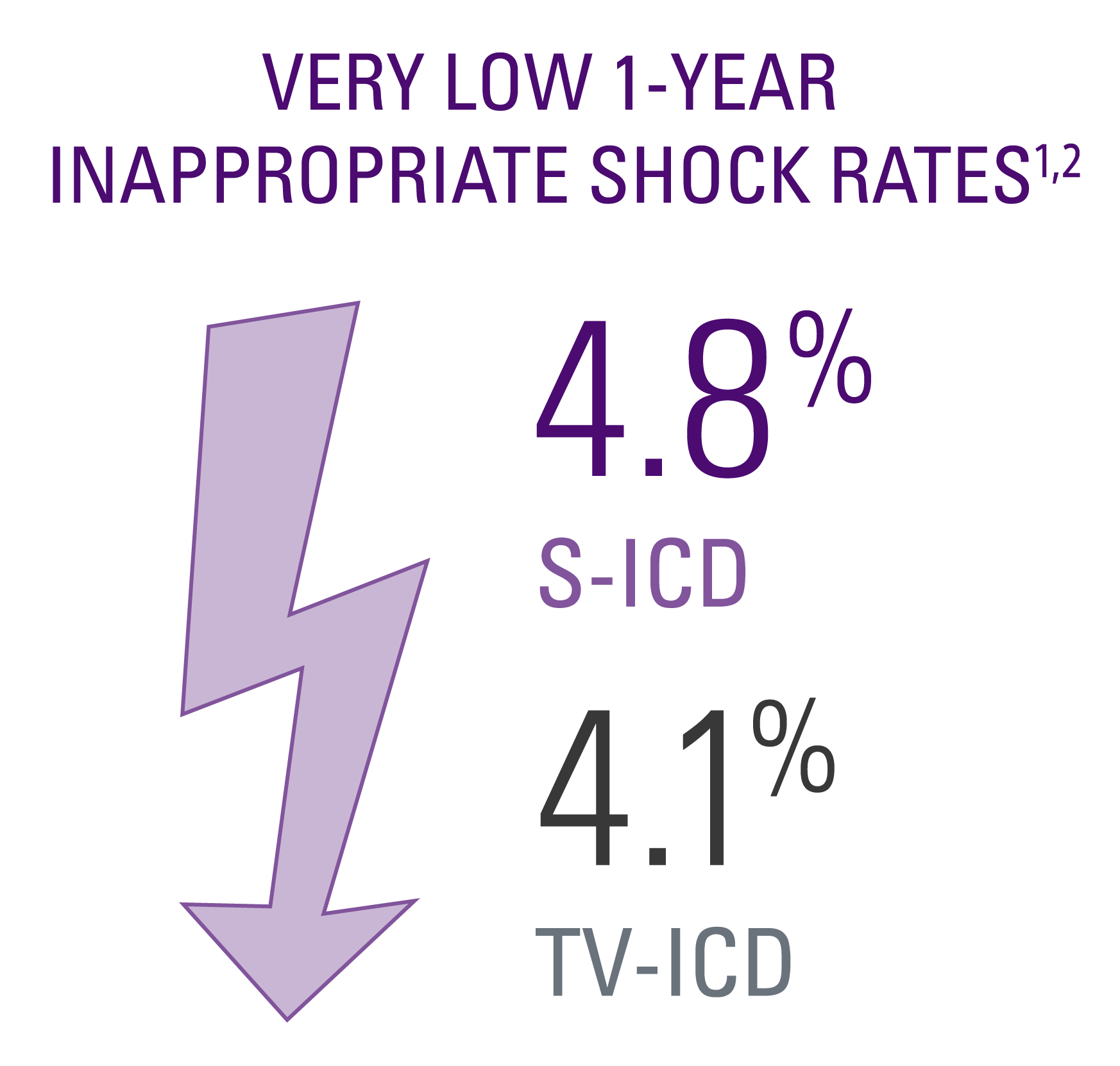
Inappropriate shock rates
- No significant difference in inappropriate shock (IAS) rates between the two groups.1,2
- The PRAETORIAN trial used mainly devices available prior to 2016. Studies using modern S-ICDs like the EMBLEMTM S-ICD have demonstrated even lower rates of IAS.5,6
S-ICD: A smart alternative to TV-ICD
Because it avoids some of the more major complications associated with the TV-ICD, including serious infection and lead-related complications, data shows that the S-ICD is an appropriate and potentially desirable alternative for primary and secondary ICD-indicated patients who do not require pacing.
Additional trial results1
- Mean implant time for S-ICD was only 5 minutes more than TV-ICD (55 minutes vs. 50 minutes implant time).
- 2-incision technique was utilized in 70% of all S-ICD implants
- Appropriate shock rates for the S-ICD group were higher than the TV-ICD group; only one patient with an S-ICD was converted to a CRT-D at two years for slow VT where ATP could potentially benefit
- Higher rates in S-ICD group likely due to: (1) devices used prior to 2016 without SMART Pass, (2) conditional programming zone of 180 bpm.5-6***
- Other contemporary S-ICD studies featured appropriate shock rates of 5.2%5 [1 yr.] in patients with a SMART Pass enabled S-ICD (comparable to 6.5% and 5.9% rates for single chamber TV-ICDs in the PainFree SST and ADVANCE III trials).7, 9

Reducing TV-ICD infections can lower mortality rates
When it comes to reducing complications, lowering mortality rates and cutting costs, avoiding infected TV-ICD leads can go a long way.
- Data in >91,000 transvenous lead extractions found that those extracted for infection had a higher overall complication rate and a higher in-hospital mortality rate compared to those without infection.4
- In this same study, the median cost of lead extraction was $39,308 for infected devices and $14,916 for non-infected devices.

Contemporary S-ICDs further reduce rates of inappropriate shock
SMART PassTM, included in the EMBLEM MRI S-ICD, has been shown to reduce IAS rates by 68%.6
- In the more recent UNTOUCHED study, the 1-year IAS rate was 3.1%,5,6 which is comparable to or lower than the rates observed with TV-ICDs in other studies, including the PRAETORIAN trial.1,7-9
- In addition, the 1-year IAS rate was 2.4% for those who received an EMBLEM MRI with SMART Pass.6
S-ICD procedures are becoming more common than ever
Optimized implant techniques (such the intermuscular technique) and implant best practices have emerged as experience with S-ICD has increased to over 150,000 patients worldwide—and counting.
First results of the randomized PRAETORIAN-DFT trial
Prospective validation of the PRAETORIAN score for prediction of defibrillation test success after subcutaneous ICD implant
The findings from this first analysis demonstrate that a low (<90) PRAETORIAN score is 99% predictive for a successful DFT and a high (≥90) PRAETORIAN score is the strongest predictor for DFT-failure (odds ratio of 34 ).1
While the study investigators concluded their presentation by stating that the PRAETORIAN score could be used as an alternative for DFT in cases when DFT-testing is not possible or desirable, omitting DFT’s in S-ICD implants cannot be advised until the full PRAETORIAN-DFT results are available.
References
PRAETORIAN XL Trial References
†Major complications were those requiring an invasive intervention.
1. Nordkamp LO. Device-related complications in transvenous versus subcutaneous defibrillator therapy during long-term follow-up: The PRAETORIAN XL Trial. HRS 2025.
2. Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al. Subcutaneous or Transvenous Defibrillator Therapy. N Engl J Med. Aug 6 2020;383(6):526-536. doi:10.1056/NEJMoa1915932.
3. Cameron Health SQ-RX PG Manual, 102098-012. 2013.
4. EMBLEM S-ICD, EMBLEM MRI S-ICD User's Manual, 92346911. 2021.
5. van der Stuijt W, et al. Heart Rhythm. Mar 2025;22(3):868-870.
PRAETORIAN Trial References
*Supported by a research grant from Boston Scientific
**Non-exhaustive
***Likely resulted in higher appropriate shock rates and total shock burden than featured in S-ICD studies where more contemporary programming and SMART Pass were utilized.5,6
1. Knops R. et al., A Randomized Trial of Subcutaneous versus Transvenous Defibrillator Therapy: The PRAETORIAN Trial. Heart Rhythm Society Late Breaking Clinical Trials LBCT-01 2020.
2. Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al. Subcutaneous or Transvenous Defibrillator Therapy. N Engl J Med. Aug 6 2020;383(6):526-536.
3. Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace. 2015;17(5):767-777.
4. Deshmukh A, Patel N, Noseworthy PA, et al. Trends in Use and Adverse Outcomes Associated with Transvenous Lead Removal in the United States. Circulation. 2015;132(25):2363-2371.
5. Theuns D, Brouwer TF, Jones PW, et al. Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2018;15(10):1515-1522.
6. Gold M. et al., Understanding Outcomes With The S-ICD In Primary Prevention Patients With Low Ejection Fraction (UNTOUCHED) Trial Primary Results. Heart Rhythm Society Late Breaking Clinical Trials LBCT-02 2020.
7. Gasparini M, Lunati MG, Proclemer A, et al. Long Detection Programming in Single-Chamber Defibrillators Reduces Unnecessary Therapies and Mortality. JACC: Clinical Electrophysiology. 2017;3:1275–82.
8. Kutyifa V, Daubert JP, Schuger C, et al. Novel ICD Programming and Inappropriate ICD Therapy in CRT-D Versus ICD Patients: A MADIT-RIT Sub-Study. Circ Arrhythm Electrophysiol. 2016;9(1):e001965.
9. Auricchio A, Hudnall JH, Schloss EJ, et al. Inappropriate shocks in single-chamber and subcutaneous implantable cardioverter-defibrillators: a systematic review and meta-analysis. Europace. 2017;19(12):1973-1980.
