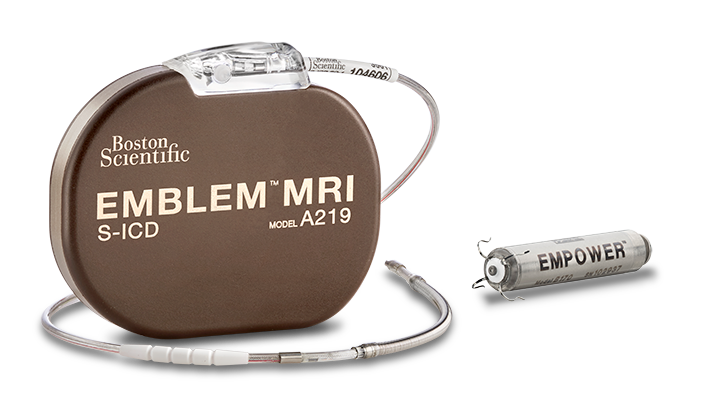
EMBLEM™ MRI S-ICD System
Subcutaneous Implantable Defibrillator

MODULAR ATP Trial: Evaluating safety, effectiveness and performance of the Modular CRM (mCRM™) System and EMPOWER™ Leadless Pacemaker (LP)
Study overview
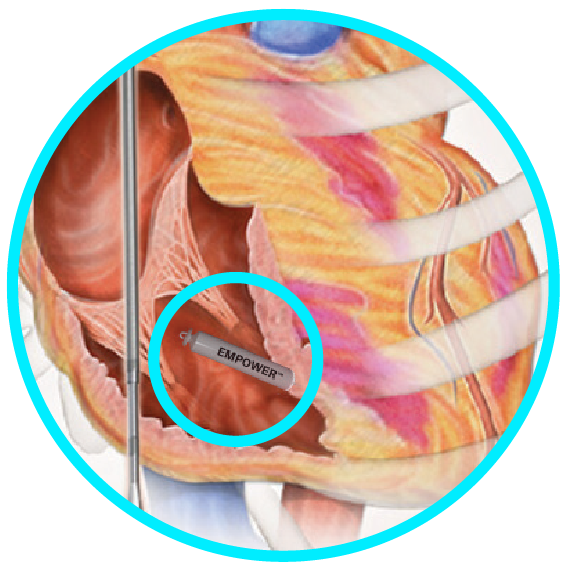
The pivotal MODULAR ATP trial is the basis for the commercialization of the industry's first Modular CRM system (mCRM) that consists of an entirely subcutaneous ICD (S-ICD) and the EMPOWER Leadless Pacemaker (LP).1,2*
The components of the mCRM system are designed to work together wirelessly to coordinate intracardiac antitachycardia pacing (ATP) therapy, provide VVIR pacing and prevent sudden cardiac death2 without the risk of leads in the heart3-5 or under the sternum.6,7
Study design
The MODULAR ATP trial is a prospective, non-randomized, single arm, global study to demonstrate the safety, performance and effectiveness of the mCRM System and the EMPOWER LP and utilized performance goals to demonstrate these primary and secondary outcomes. In addition to evaluating the safety of the EMPOWER LP System, the MODULAR ATP trial evaluated the ability of the EMBLEM S-ICD to successfully communicate a wireless request to the EMPOWER LP to deliver intracardiac ATP.1,** Specific testing was also conducted to determine the performance and effectiveness of the EMPOWER LP to function as a standalone VVIR pacemaker.2,†
Enrollment
From July 2021 through January 2024, 293 patients were enrolled at 38 centers. The study design included a pre-specified early analysis of the safety endpoint once 134 patients were implanted with the EMBLEM S-ICD and EMPOWER LP System and followed for 6 months. Because of variability in the 6-month follow up appointments, 162 patients were in this group for this early analysis of the safety endpoint. The mean age of this group was 60 years with a mean EF of 33%. 61% had ischemic cardiomyopathy and 54% were primary prevention patients. 1,8
The MODULAR ATP trial enrolled patients with significant structural heart disease and implantation of the EMPOWER LP was successful in 100% of patients.1,8

Results1
With 162 patients implanted, the mCRM System achieved all pre-specified safety and effectiveness 6-month endpoints from the MODULAR ATP trial. These results demonstrated the safety profile of the EMPOWER LP system.

Intracardiac ATP
The EMPOWER LP is designed to deliver intracardiac ATP and brady pacing therapy that should not lead to pain or discomfort in contrast to extracardiac pacing.1,8,9 ATP conversion success rate was 61.3% and no patient in the MODULAR ATP trial requested leadless pacemaker therapy inactivation due to feelings of pain or discomfort.1,8,‡
MODULAR ATP 12-month trial data
- The EMPOWER LP System demonstrated a consistent complication free rate of 97.2% at 12 months following implant (n = 286).10
- For patients who needed ATP in the MODULAR ATP trial, the mCRM System devices and therapy demonstrated effectiveness to pace-terminate VA in 65.4% of episodes, resulting in painless ATP8 and shock-free arrhythmia termination in almost half of discrete VA episodes.11
- The EMPOWER LP withheld‡‡ ATP therapy in 19% of treated episodes; 43% of which were inappropriately S-ICD sensed events.11,12
- These data demonstrate that the mCRM System reduced the shock burden‡‡ by an estimated 56% within the MODULAR ATP patient population compared to an S-ICD in isolation.12
Designed for the future of personalized patient care
Upon the EMPOWER LP and mCRMTM system receiving FDA approval, EMPOWER will be the first and only LP designed to be a standalone VVIR pacemaker that is compatible with all existing EMBLEMTM S-ICD devices as part of the mCRM system. The mCRM system is designed to deliver painless intracardiac ATP and/or brady pacing.2
Should patients currently implanted with an EMBLEM S-ICD device develop a need for ATP and/or bradycardia pacing, an upgrade pathway will be available once the EMPOWER LP and mCRM system receive FDA approval.2
The mCRM system is designed to provide upgrade pathways regardless if the EMBLEM S-ICD or EMPOWER LP is implanted first, providing physicians flexibility to tailor therapy to the individual patient’s needs.2

The New England Journal of Medicine article:
A Modular Communicative Leadless Pacing–Defibrillator System
Pacing Performance of EMPOWER™ LP
.png)
Pacing parameters13
EMPOWER has consistent, stable pacing performance over time:
- Thresholds < 0.6 ± 0.5 V
- Sensed amplitude > 14 ± 6 V
- Pacing impedance ~ 700 ± 130 Ω
Prof. Knops 2024 HRS presentation


APPRAISE ATP: Assessment of primary prevention patients receiving an ICD-systematic evaluation of ATP
Study design and overview14
The APPRAISE ATP trial was a prospective, randomized, global, multicenter clinical trial to understand the role of ATP in primary prevention (PP) patients currently indicated for ICD therapy.
The objective of the trial was to determine if standard therapy (ATP prior to shock) is clinically equivalent to No ATP prior to shock when combined with contemporary programming in primary prevention TV-ICD indicated patients. The trial was designed to determine if programming ATP OFF in Zone 2 in PP patients is equivalent to programming ATP ON by measuring time to first all-cause shock.
Enrollment and randomization14,15
The APPRAISE ATP trial is the largest head-to-head trial of ATP in primary prevention patients. The trial enrolled 2,626 primary prevention ICD indicated patients from 134 centers in North America, Europe, and Asia.15 Patients received a single- or dual-chamber transvenous ICD (TV-ICD) and 2,595 were randomized 1:1 to different therapy programming in an equivalence study design with sequential superiority analysis of each arm.
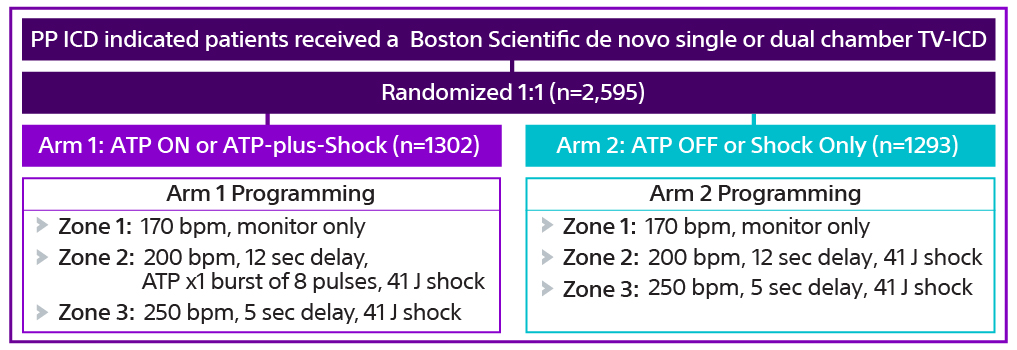
Results15
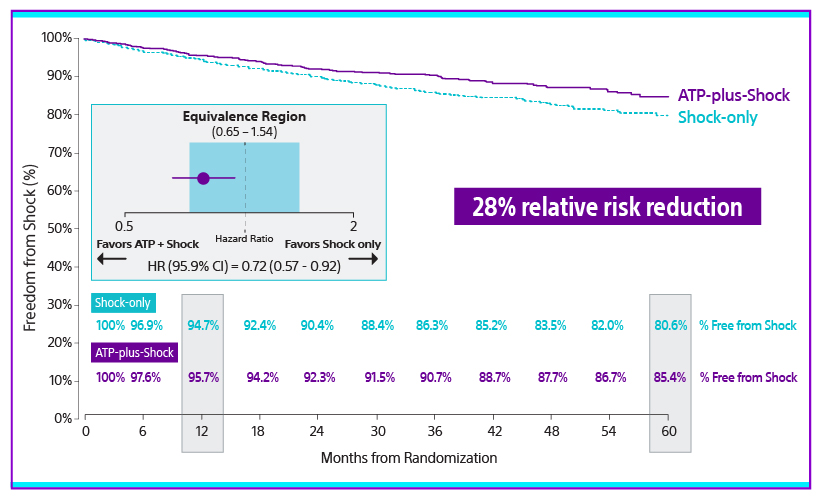
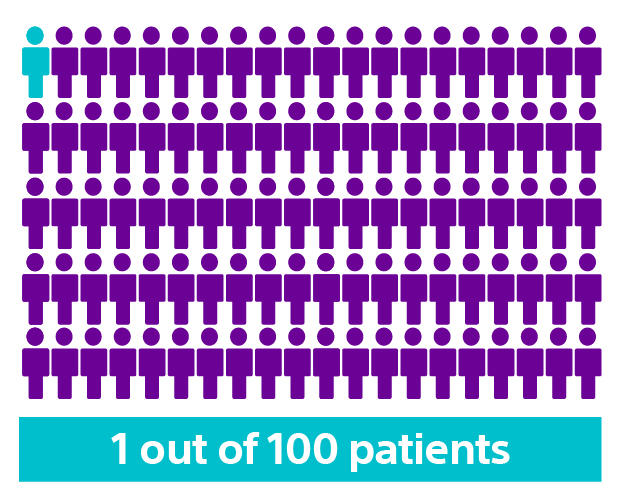
The APPRAISE ATP trial demonstrated superiority with a 28% relative risk reduction in time to first all-cause shock for the ATP ON arm compared to the ATP OFF arm (Log-rank P-value=0.005). This represents an absolute all-cause shock reduction in 1% of PP ICD indicated patients per year.
There was a significant difference throughout follow up (p=0.005). The percentage of patients free from all-cause shocks at one year was 95.7% for ATP-plus-shock arm vs 94.7% for the shock-only arm.
At five years, the percent of patients free from all-cause shocks was 85.4% for the ATP-plus-shock arm vs 80.6% for the shock-only arm.15
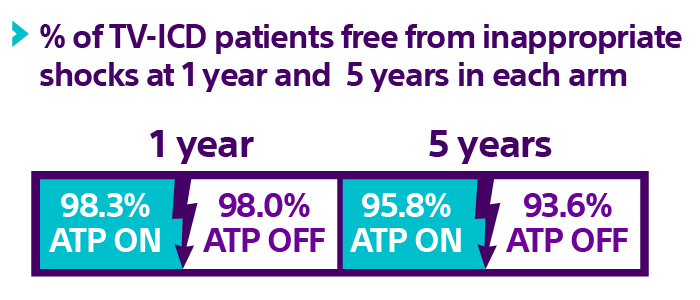
Inappropriate shock rates low in both arms
Full follow-up results
The importance of shared decision making
Journal of the American Medical Association:
Assessment of Antitachycardia Pacing in Primary Prevention Patients
Dr. Schuger 2024 HRS presentation
Future upgrade pathway with the Modular CRM (mCRM™) System*
Should patients currently implanted with an EMBLEM S-ICD device develop a need for intracardiac ATP and/or bradycardia pacing, an upgrade pathway will be available once the EMPOWER LP* and mCRM system receive FDA approval.
The mCRM system is designed to provide upgrade pathways regardless if the EMBLEM S-ICD or EMPOWER LP is implanted first, providing physicians flexibility to tailor therapy to the individual patient’s needs.2
* The EMPOWER leadless pacemaker and mCRM system will pursue FDA approval in 2025. Caution: Investigational Device. Limited by US law to investigational use only. Not available for sale.
** The mCRM wireless communication is inductive, one-way communication from the S-ICD to the LP to request ATP delivery. Communication testing, which assessed pass/fail of communication at the programmed S-ICD Telemetry Setting, was required at 6-month visit in 4 body postures. The test had to pass in > 88% of communication attempts, across all postures, to be defined as successful.
† Rate-response results will be reported in a future publication.
‡ ATP success rate defined as terminating ventricular arrhythmia.
‡‡ Shocks were avoided by (1) delivering ATP OR (2) LP withholding ATP and the S-ICD transitioning to a conservative sensing strategy.12
§ Defined as all shocks over full follow-up period per 100 patients.
References
1. Knops R. Tachycardia Therapy and Trial Endpoint Results of the First Modular, Intra-body, Communicating Subcutaneous Defibrillator-Leadless Pacemaker System: MODULAR ATP Interim Cohort. Heart Rhythm Society Late Breaking Clinical Trials. May 18th, 2024 LB-469805-03
2. Lloyd MS, Brisben AJ, Reddy VY, et al. Design and rationale of the MODULAR ATP global clinical trial: A novel intercommunicative leadless pacing system and the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm O2. Jul 2023;4(7):448-456. doi:10.1016/j.hroo.2023.05.004
3. Knops RE, Pepplinkhuizen S, Delnoy P, et al. Device-related complications in the subcutaneous and transvenous ICD: a secondary analysis of the PRAETORIAN trial. Eur Heart J. Aug 28 2022;43(47):4872-4883. doi:10.1093/eurheartj/ehac496
4. Healey JS, Krahn AD, Bashir J, et al. Perioperative Safety and Early Patient and Device Outcomes Among Subcutaneous Versus Transvenous Implantable Cardioverter Defibrillator Implantations : A Randomized, Multicenter Trial. Ann Intern Med. Nov 8 2022;175(12):1658-1665. doi:10.7326/M22-1566
5. Leong D DH, Mondesert BA et al. Effects of Implantable Cardioverter-Defibrillator Leads on the Tricuspid Valve and Right Ventricle: A Randomized Comparison of Transvenous versus Subcutaneous Leads. Heart Rhythm Society Late Breaking Clinical Trials. May 19th 2023;LB-456090-02
6. Payne JE, Gold MR. A Substernal Defibrillator Lead With Pacing Capability: Another Tool in the Toolbox? JACC Clin Electrophysiol. Feb 2019;5(2):197-198. doi:10.1016/j.jacep.2018.12.006
7. Romers H, van Dijk V, Boersma L. Evolution of extravascular implantable cardioverter-defibrillator therapy for ventricular arrhythmias. Heart Rhythm O2. Jan 2023;4(1):59-64. doi:10.1016/j.hroo.2022.09.021
8. Knops RLM, Roberts PR, et al. A Modular Communicative Leadless Pacing-Defibrillator System. N Engl J Med. 2024;doi:0.1056/NEJMoa2401807
9. Friedman P, Murgatroyd F, Boersma LVA, et al. Efficacy and Safety of an Extravascular Implantable Cardioverter-Defibrillator. N Engl J Med. Oct 6 2022;387(14):1292-1302. doi:10.1056/NEJMoa2206485
10. Lloyd, MS et al. One year tachycardia/bradycardia functionality from the MODULAR ATP trial. HRS 2025 Scientific Session, PO-07-193
11. Doshi, MS et al. Ventricular arrythmia detections and therapy in the ongoing MODULAR ATP trial: A Modular, communicative, leadless pacing-defibrillator system. HRS 2025 Scientific Session, PO-06-021
12. Lloyd, MS et al. Shock avoidance in spontaneous arrythmia episodes by a modular, communicative, leadless pacing-defibrillator system (MODULAR ATP trial). HRS 2025 Scientific Session, PO-FP-035
13. Mont L. et al. Pacing Performance of the First Leadless Pacemaker Communicating with an S-ICD from the full cohort of the MODULAR ATP study. European Society of Cardiology Late Breaking Clinical Trials and Science, September 2, 2024.
14. Schuger CD, Ando K, Cantillon DJ, et al. Assessment of primary prevention patients receiving an ICD - Systematic evaluation of ATP: APPRAISE ATP. Heart Rhythm O2. Aug 2021;2(4):405-411. doi:10.1016/j.hroo.2021.07.003
15. Schuger C, Joung, B., Ando, Kl, et al. Assessment of Primary Prevention Patients Receiving An ICD- Systematic Evaluation of ATP: APPRAISE ATP. Heart Rhythm Society Late Breaking Clinical Trials. May 17th, 2024 LB-469803-02
16. Schuger C, Joung B, Ando K, et al. Assessment of Antitachycardia Pacing in Primary Prevention Patients: The APPRAISE ATP Randomized Clinical Trial. JAMA. Published online October 3, 2024. doi:10.1001/jama.2024.16531.
17. Wilkoff BL, Fauchier L, Stiles MK, et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. Feb 2016;13(2):e50-86. doi:10.1016/j.hrthm.2015.11.018
18. Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al. Subcutaneous or Transvenous Defibrillator Therapy. N Engl J Med. Aug 6 2020;383(6):526-536. doi:10.1056/NEJMoa1915932




