EMBLEM™ S-ICD System
Subcutaneous Implantable Defibrillator
Why mCRM therapy?
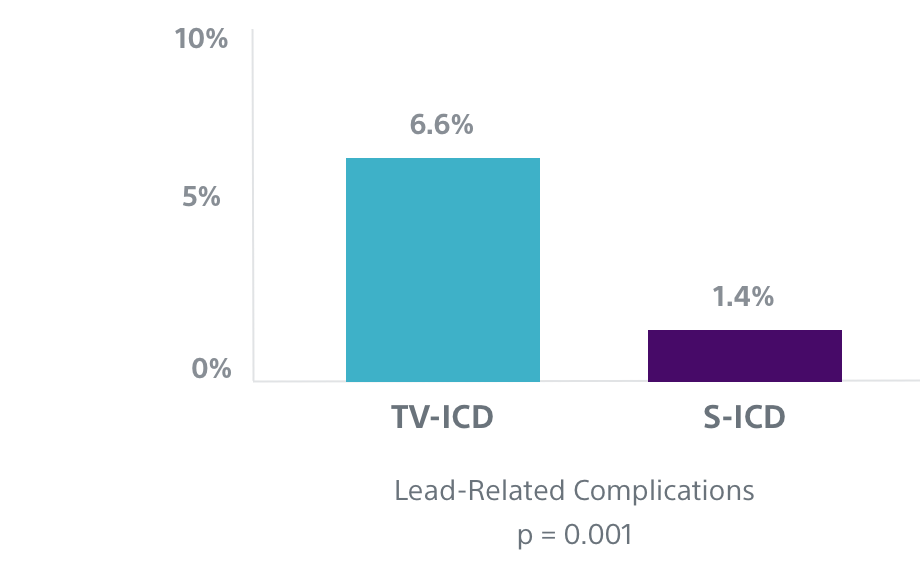
Fewer lead-related complications seen in the PRAETORIAN trial3
The EMBLEM S-ICD leaves the vasculature untouched, thereby reducing the risk of acute and future complications associated with transvenous leads.
In the first prospective randomized head-to-head trial comparing the performance of S-ICD and TV-ICD, data showed that TV-ICD patients experienced more than four times as many lead-related complications as S-ICD patients.3
EMPOWER™ LP procedure videos
How mCRM therapy works
1. When the EMBLEM S-ICD senses an episode of tachycardia, it triggers the EMPOWER LP to provide ATP therapy, which may stop the tachycardia and reset the heart to a normal rhythm.
2. The EMBLEM S-ICD continuously monitors for the result of the ATP therapy.
a. If the ATP successfully terminated the tachycardia and returned the heart to a normal rhythm, the EMBLEM S-ICD will continue monitoring and no shock will be delivered.
b. If the ATP did not terminate the tachycardia, the EMBLEM S-ICD will deliver shock therapy.
Example of mCRM Therapy
(Tjong, F.V.Y., et al. HRS 2016)
Watch the video to see the coordinated therapy between the EMBLEM S-ICD and EMPOWER LP during a mCRM therapy sequence.
mCRM system components
EMBLEM S-ICD
The only extrathoracic implantable defibrillator that provides protection from both sudden cardiac death and the risks and complications associated with transvenous leads.
- Eliminates potential for vascular injury, transvenous lead insertion complications, lead-associated tricuspid regurgitation, mechanically induced pro-arrhythmia, and transvenous lead failure and associated extraction risk
- Reduces risk of systemic infection
- Preserves the vasculature
- Remains outside the ribcage, never touching the heart

EMPOWER LP*
The EMPOWER LP is designed to be paired with S-ICD to provide pacing or ATP therapies at the time they are needed.
- ATP when commanded by a paired S-ICD
- Delivery system with inner extendable shaft
- 20.7 F delivery catheter
- Dedicated retrieval catheter
- Rate response via accelerometer
- > 10 Year average longevity expected when used primarily for ATP therapy as part of an mCRM System
Are You a Clinical Site Investigator?
All EMBLEM S-ICDs can be upgraded and paired with EMPOWER Leadless Pacemakers
*Caution: Investigational Device. Limited by US law to investigational use only. Not available for sale.
Products shown for informational purposes only. Not meant as a promotion or offer for sale. Certain components are pending CE Mark. Not available for sale in the European Economic Area (EEA).
References
1. Gasparini, M, Lunati, MG, Proclemer, A, et al., Long Detection Programming in Single-Chamber Defibrillators Reduces Unnecessary Therapies and Mortality. JACC: Clinical Electrophysiology, 2017.
2. Data on VR devices from LATITUDE Boston Scientific data on file.
3. Knops R. et al., A Randomized Trial of Subcutaneous versus Transvenous Defibrillator Therapy: The PRAETORIAN Trial. Heart Rhythm Society Late Breaking Clinical Trials LBCT-01 2020.
4. Botto, GL. The Italian subcutaneous implantable cardioverter-defibrillator survey: S-ICD, why not? EUROPACE. Dec 2016.



.jpg)
