The economic impact of reducing Target Lesion Revascularisation
EluviaTM Drug-Eluting Stent demonstrates improved patient outcomes by reducing both TLR rates and associated costs, thereby impacting the healthcare system.
TLR Highlights: Fewer repeated procedures with EluviaTM

At 2 years, Eluvia Drug-Eluting Stent
offers a significant reduction (70%) in
Target Lesion Revascularisation
(repeat interventions).1
Economic Highlights: Better patient outcomes at a lower cost from
COSTLY-TLR Study
The study shows the overall cost of a TLR2 in the femoropopliteal artery: ~22K € *
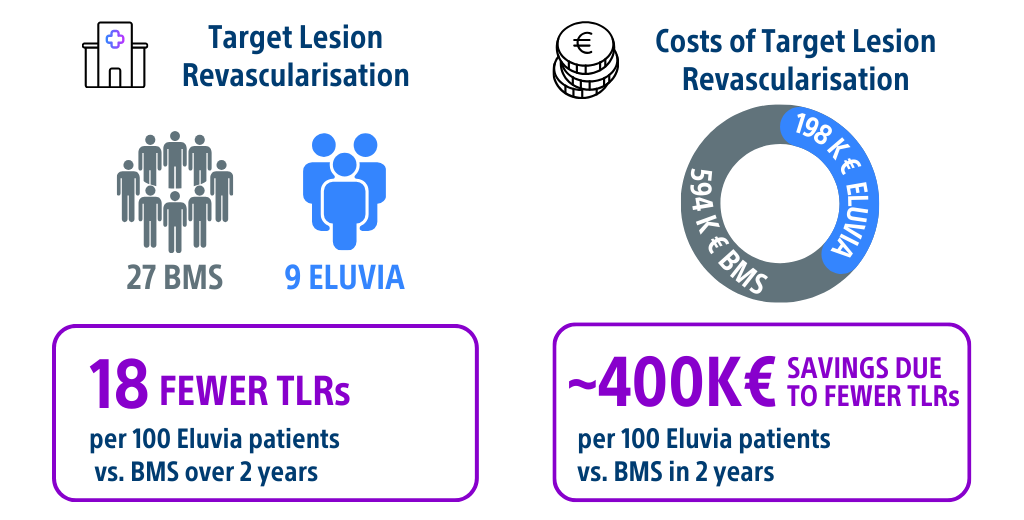
- 18 fewer TLRs per 100 Eluvia patients vs. BMS over 2 years.
- ~ 400K € savings due to fewer TLRs per 100 Eluvia patients vs. BMS in 2 years.
*Underestimation, considering only the operating time cost that is the main driver of overall TLR costs
Do you want to get more information on COSTLY-TLR Study?
COSTLY-TLR Study
The COSTLY-TLR Study2 defines and reports the real-world costs of target lesion revascularisation procedures (TLR) in patients with femoropopliteal in-stent-restenosis and occlusion (ISR) following stenting, from a healthcare payer’s perspective.
Study overview
- European multicenter study
- 6 countries (UK, France, Belgium, Germany, Italy, Spain) – 13 centers
- 482 TLR procedures included
(52% hybrid procedures, 42% endovascular procedures, 6% open surgery procedures)
Study conclusions
- The costs of repeated revascularisations are not neglectable, and the initial strategy should aim to achieve better patency, as the expenses of a secondary procedure are particularly high.
- It has been identified the mean total cost of a TLR (for all procedures):
~22K € * – procedural time was the main driver of the periprocedural costs associated with TLR. - The results provide justification for investigating and using techniques or types of intervention associated with reduced TLR when dealing with femoropopliteal peripheral artery disease.
*Underestimation, considering only the operating time cost that is the main driver of overall TLR costs
References:
1. Goueffic ,Y. CIRSE 2023 adapted from Katsanos K et al. Economic analysis of endovascular drug-eluting treatments for femoropopliteal artery disease in the UK. BMJ Open. 2016 May 9;6(5):e011245. doi: 10.1136/bmjopen-2016-011245. PMID: 27160845; PMCID: PMC4874117.
2. Saratzis et al. COST Analysis of Target Lesion Revascularisation in Patients With Femoropopliteal In-Stent Restenosis or Occlusion: The COSTLY-TLR Study. Eur J Vasc Endovasc Surg. 2024 Feb 6:S1078-5884(24)00160-6. doi: 10.1016/j.ejvs.2024.02.001. Epub ahead of print. PMID: 38331163.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.