
Majority of ACURATE neo2 implants were appropriately expanded with a mid-frame under expansion identified in a small proportion of patients 9.4% (n=57) vs. the ACURATE IDE trial (21.3%).

When post dilation was carried out, it was effective in reducing the rate of mid-frame under expansion in 92% of cases (45/49).

The composite rate at one year was 37.6% in the mid frame under expansion arm 10.2% in the expanded arm. (p<0.01).
The PROVE ACURATE neo2 Registry
A Prospective, multicenter registry of midterm outcomes with ACURATE neo2 in up to 30 EU centers enrolling N=~1000 patients.
See below the main 30-day outcomes (VARC 3) presented at EuroPCR 2024 by Prof. Holger Thiele or Mohammad Abdel-Wahab.

All-cause mortality

Transvalvular gradient at discharge (mmHg)

Pacemaker rates
ACURATE neo2 PMCF Post-market Registry| 1-year outcomes
A prospective multicenter single-arm post-market surveillance study for ACURATE neo2 in a routine clinical practice setting.
5.1%
mortality rate
0.9%
>moderate PVL
8mmHg
mean gradients
Early neo2 Registry core-lab results
First large, real-world clinical data on the ACURATE neo2™ Aortic Valve System: The Early neo2 Registry
Independently studying 85% of the original study’s 554 patients, the Early neo2 Registry core-lab analysis confirmed minimized paravalvular leak rates with the ACURATE neo2 Valve and its Active PVsealTM sealing skirt.
6.0%
new permanent pacemaker
2.7%
moderate to severe PVL1
8.0mmHg
mean gradients
COMALIGN neo2 Study
A retrospective study of 900 patients implanted with ACURATE neo2 across 3 European sites. This is the largest study of commissural alignment to date, and one of the first assessments of TAVI valve performance based on the degree of commissural alignment.
95%
Of cases avoided severe commisural misalignment 4
- Severe commissural misalignment is associated with an increased risk of severe Patient Prosthesis Mismatch (PPM).
- Total aortic regurgitation was significantly higher for patients with severe commissural misalignment.
COMALIGN Study
First real-world analysis of patient-specific implantation techniques using different devices. The COMALIGN study demonstrated that neo-commissural alignment and optimized valve design can improve procedural success.
Best-in-class commissural alignment: ACURATE neo2 was the only self-expanding valve to 100% avoid commissural misalignment.2

0% moderate or severe commissural misalignment
ACURATE neo2 Valve
20/20 successful consecutive cases
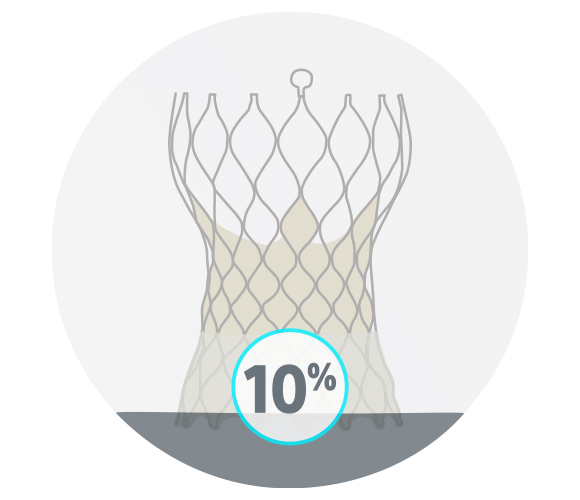
10% moderate or severe commissural misalignment
Evolut™ R/PRO Valve
17/20 successful consecutive cases; 3 cases with non-optimal rotations
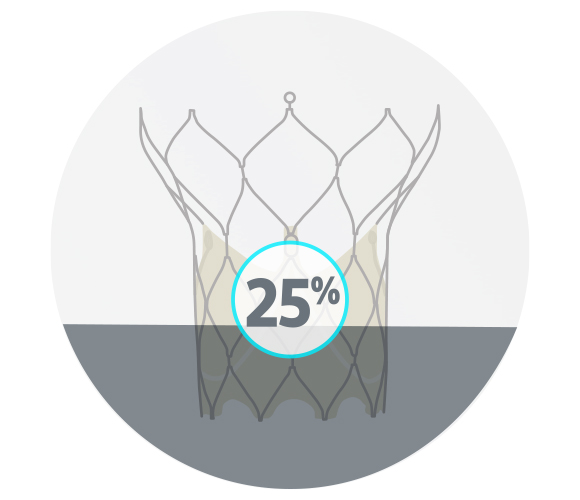
25% moderate or severe commissural misalignment
PORTICO™ Valve
15/20 successful consecutive cases; 5 cases difficult to assess
RE-ACCESS 2 Study
This trial studied coronary re-engagement in patients undergoing transfemoral TAVI with Evolut R/PRO/PRO+ or ACURATE neo2 implanted with patient specific commissural alignment technique.
Large cell frame design remains the most impactful factor in coronary re-engagement post TAVI.
5.5%
unsuccessful coronary cannulation (7 patients)
6 patients received Evolut5

RE-ACCESS Study
First systematically evaluated analysis of coronary access before and after TAVI in an all-comers’ population.
With zero incidences of unsuccessful coronary cannulation, ACURATE neo demonstrated an extremely high coronary access success rate.
82%
Evolut™ R/PRO
82% successful coronary cannulation
n = 1233
100%
ACURATE neo™ Valve Platform
100% successful coronary cannulation
n = 723
99%
SAPIEN Platform
99% successful coronary cannulation
n = 963
REFERENCES
1. Möllmann H, Holzhey DM, Hilker M, et al. The ACURATE neo2 valve system for transcatheter aortic valve implantation: 30-day and 1-year outcomes. Clin Res Cardiol. 2021;110(12):1912–1920.
2. Bieliauskas G, Wong I, Bajoras V, et al. Patient-specific implantation technique to obtain neo-commissural alignment with self-expanding transcatheter aortic valves. J Am Coll Cardiol 2021;14(19):2097-2108.
3. Reobtain Coronary Ostia Cannulation Beyond Transcatheter Aortic Valve Stent (RE-ACCESS); NCT04026204 J Am Coll Cardiol Intv. 2020.
4. De Backer O. Commissural alignment & TAV performance: Results from the COMALIGN-neo2 study. TCT 2023
5. Costa G. The REACCESS-2 Study. PCRLV 2023
The ACURATE IDE LBCT, TCCT 2024, Dr. M. Reardon
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France. Product available in the European Economic Area (EEA) only. Please check availability with your local sales representative or customer service
















