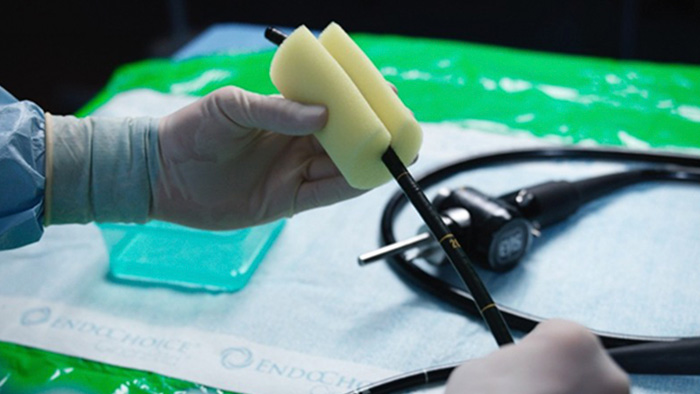
Eliminating the risk of contaminated equipment is vital
Reusable equipment, such as valves and scopes, can show contamination after reprocessing and may be the source of infections if cleaning, drying, storage, and hand hygiene are inadequate.
For example, studies show that bacteria, spores, yeast, and/or mold can be found on up to 50% of cleaned valves.1,2
In addition, reusable scopes can be fragile. Their maintenance can be costly, with frequent repairs and labor-intensive cleaning and sterilization requirements.3,4
Long guide wires may also pose a risk as their length makes them vulnerable to accidental contact with unclean surfaces.5
Watch as experts discuss a breakthrough in infection prevention
A range of materials, optimized for infection prevention

Our single-use valves and scope systems deliver:
- Consistent, predictable performance
- Guaranteed sterility that eliminates the risk of cross-contamination
- Flexibility for night and/or weekend procedures
The Boston Scientific single-use portfolio eliminates duodenoscope reprocessing, servicing, and repairs. It also minimizes the risk of infection, and its associated costs.
The 260 cm guidewires of the Boston Scientific short wire system eliminate the risk of accidentally touching the floor.
Explore our range of intra-procedural infection prevention products:


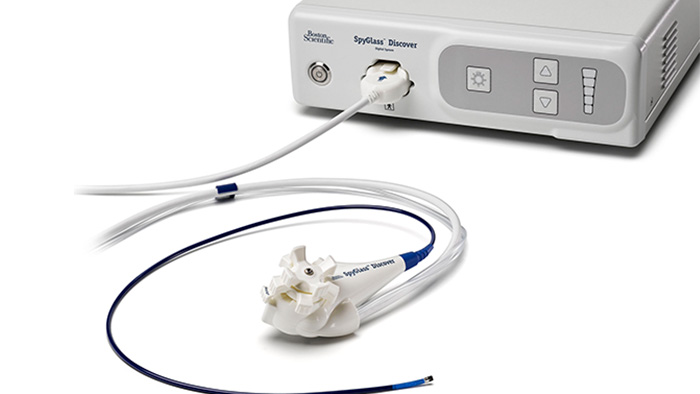
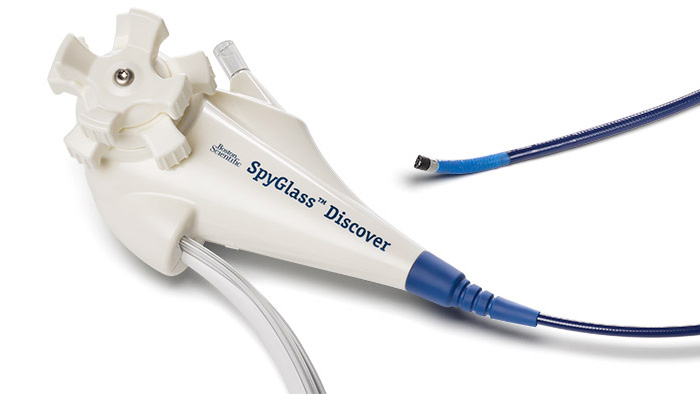
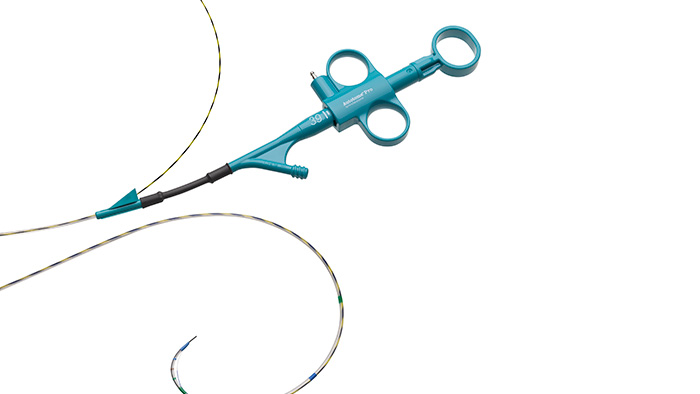
Facilitating infection prevention in the HPB (hepato-pancreato-biliary) pathway
At Boston Scientific, we understand the array of obstacles you face in ensuring that your patients undergoing ERCP avoid infection. That is why we have developed solutions for every step in the process.