CHAMPION-AF Clinical Trial
Why CHAMPION-AF?
Currently, WATCHMAN FLXTM is an FDA-approved device alternative for AFib patients who are unable to tolerate long-term blood thinner medication.
The CHAMPION-AF Clinical trial is studying WATCHMAN FLX as a first choice option versus blood thinners for AFib patients who are able to tolerate long-term blood thinner use, but would consider a one-time, device option for stroke risk reduction.
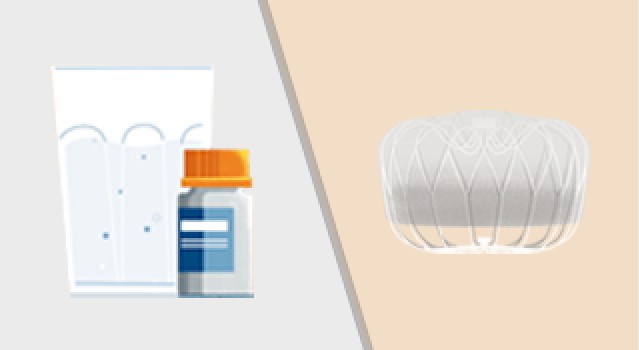
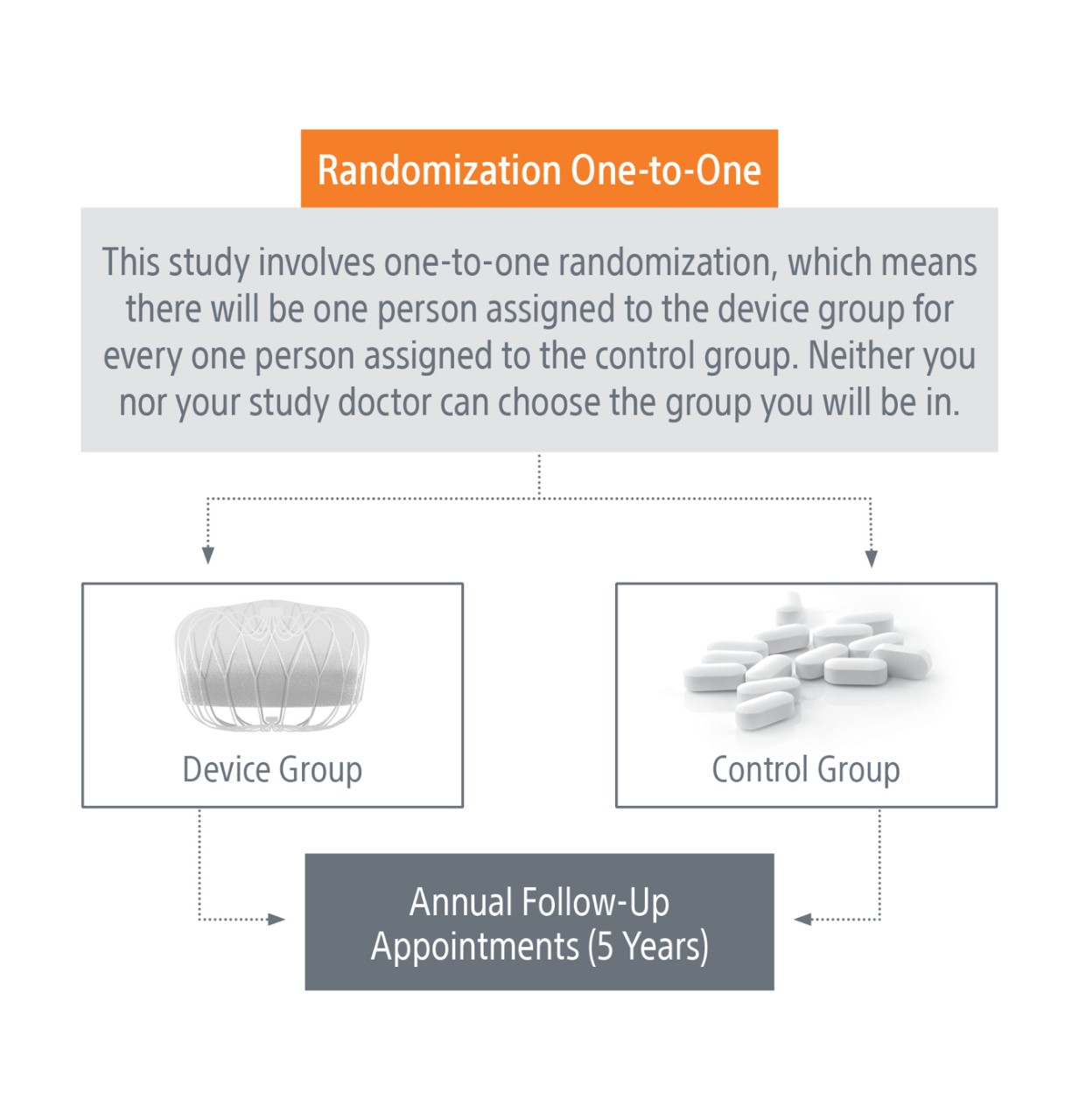
If you decide to participate in this study, you will be “randomized” into either the “device” or “control” group. Randomization means that you are put into a group by chance, like the flip of a coin. Candidates randomized to the device group will be scheduled for a WATCHMAN FLX Implant. Control group candidates will not have the device implanted and will be prescribed a blood thinner (NOAC) for the duration of the trial at the discretion of the study doctor.
Who May Qualify for CHAMPION-AF?
What is Required of Me in the Trial?

By joining this study:
- You will be randomized to either the device group OR the NOAC group.
- You will be expected to visit your study doctor for required screenings.
- You will be required to attend follow‑up visits once a year, for up to five years. Some follow‑up appointments may be available virtually.
Is the CHAMPION‑AF Trial Right for Me?
Talk to Your Doctor
For more information, or to find out if you're an appropriate candidate for the CHAMPION‑AF clinical trial, fill out and print the simple trial discussion guide below, and bring it to a scheduled appointment with your heart doctor.
* All mentions of 'AFib' or 'Atrial Fibrillation' specifically indicate atrial fibrillation, not caused by a heart valve problem.
1. Kabra R, Cram P, Girotra S, Vaughan Sarrazin M. Effect of Race on Outcomes (Stroke and Death) in Patients >65 Years with Atrial Fibrillation. Am J Cardiol. 2015;116:230–235. doi: 10.1016/j.amjcard.2015.04.012.
Please talk to your physician about any additional risks related to the CHAMPION-AF trial.
Important Safety Information
The WATCHMAN and WATCHMAN FLX Devices are permanent implants designed to close the left atrial appendage in the heart in an effort to reduce the risk of stroke.
With all medical procedures there are risks associated with the implant procedure and the use of the device. The risks include but are not limited to accidental heart puncture, air embolism, allergic reaction, anemia, anesthesia risks, arrhythmias, AV (Arteriovenous) fistula, bleeding or throat pain from the TEE (Trans Esophageal Echo) probe, blood clot or air bubbles in the lungs or other organs, bruising at the catheter insertion site, clot formation on the device, cranial bleed, excessive bleeding, gastrointestinal bleeding, groin puncture bleed, hypotension, infection/pneumonia, pneumothorax, pulmonary edema, pulmonary vein obstruction, renal failure, stroke, thrombosis and transient ischemic attack. In rare cases death can occur.
Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of the device.