What is the difference between a pacemaker and a defibrillator?
Two common devices used to control the heart’s rhythm are pacemakers and implantable cardioverter defibrillators (ICD). Both devices use electrical impulses to stimulate the heart. However, there are distinct differences between them:
Pacemaker
- A pacemaker helps control an abnormally slow heartbeat
- A pacemaker sends small electrical pulses to the heart if the heart is beating too slow
Defibrillator
- An ICD device sends an electric shock to the heart if it detects a dangerously fast heartbeat to restore the heart to its normal rhythm
- An ICD is designed to prevent sudden cardiac death
Learn about ICD options
If your doctor has recommended an ICD for you, you’ll have a couple of decisions to make. First, do you want to get a device? And if so, which type of device? This page and conversations with your doctor can help you learn more about your options so you can make the right decision for you.
This process is called Shared Decision Making. To learn more about what Shared Decision Making means for you as a patient, watch a 30-minute educational program on this topic offered by Mended Hearts. Mended Hearts is the nation’s premier peer-support program for patients who have cardiovascular disease, their caregivers, and their families.
Why is my doctor recommending a device?
Your doctor will share details about your specific medical condition. But in general, ICDs are used to protect people from sudden cardiac death. Sudden cardiac arrest happens when the electrical system in your heart stops working. That means your heart stops pumping blood to your body. Without treatment, someone experiencing sudden cardiac arrest can die within minutes. That is called a sudden cardiac death.
Only 1 in 10 people who have sudden cardiac arrest outside the hospital without an ICD survive.1 With an ICD >95% live.2,3
How does an ICD work?
An ICD will monitor your heart rhythm. If it detects an abnormal life-threatening rhythm – called an arrhythmia – it will deliver a shock to restore your heart’s normal rhythm. Some patients say having an ICD is like having a paramedic with them at all times. Depending on your medical condition and type of heart rhythm, your doctor may recommend an ICD that provides a type of pacing called ATP, or anti-tachycardia pacing. While not painful, some patients report they can feel the ATP being delivered. In some patients, ATP can be used instead of a shock to restore their heart’s normal rhythm.
Can an ICD prolong my life?
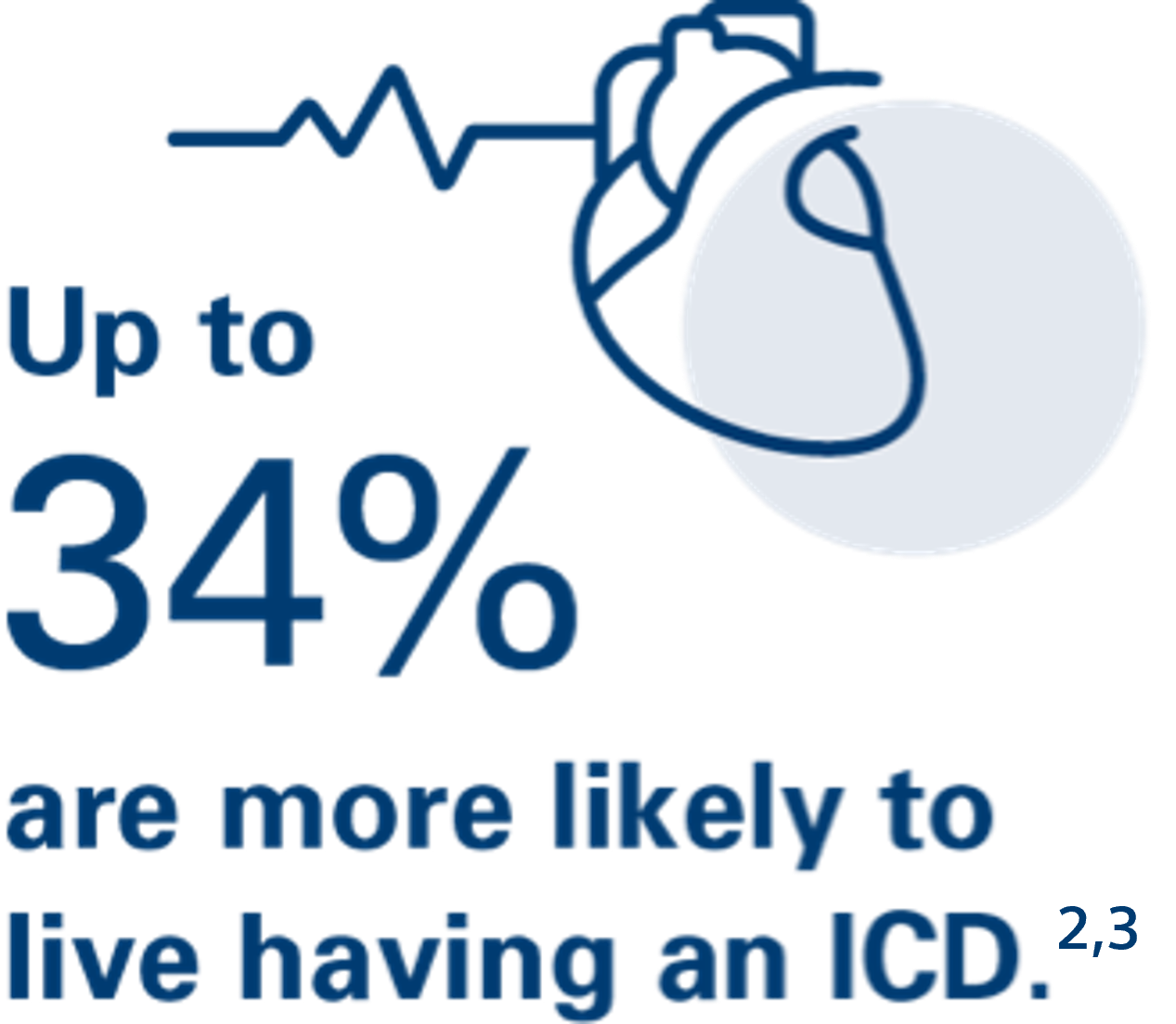
In studies of people at risk for sudden cardiac arrest, those with an ICD were 23%–34% more likely to live compared to people without an ICD.2,3
Do I want a device?
Only you and your doctor can make that decision. But here’s some information that may help you decide.
Learn more about your treatment options
Types of defibrillators
There are two types of Boston Scientific defibrillators: Subcutaneous ICD (S-ICD) and Transvenous ICD (TV-ICD). The main difference between the two devices is where the lead – that’s the wire that will deliver the shock to your heart – is placed. With a TV-ICD, the lead is placed inside the heart. An S-ICD’s lead does not touch the heart; it is placed under the skin on your chest (refer to the pictures below). Your doctor will talk with you about the defibrillator options and together you will decide what is the optimal choice for you.
EMBLEM™ MRI S-ICD and TV-ICD
ICD therapy is a very trustworthy therapy that has prolonged hundreds of thousands of lives. When ICD devices were first introduced in the 1980s, they were implanted in the abdomen. Later came the transvenous ICD (TV-ICD) which is implanted in the left chest area near the collarbone. The less invasive subcutaneous (under the skin) ICD system, or EMBLEM MRI S-ICD System, delivers protection without touching the heart.
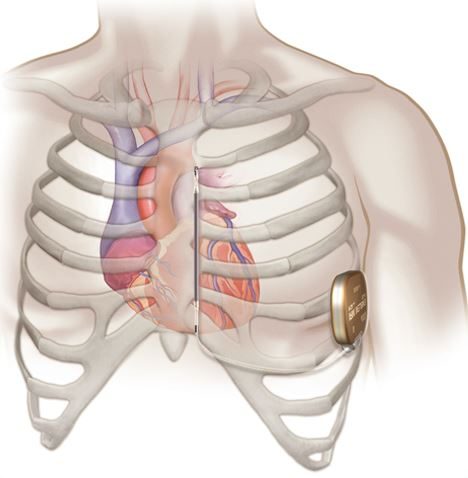
EMBLEM MRI S-ICD system implant procedure
- Unlike a transvenous ICD device, the EMBLEM MRI S-ICD is typically implanted on the left side of the chest next to the rib cage and the lead is implanted just under the skin next to the breastbone.
- The EMBLEM MRI S-ICD System delivers therapy without the need for wires implanted in the heart or under the ribcage.
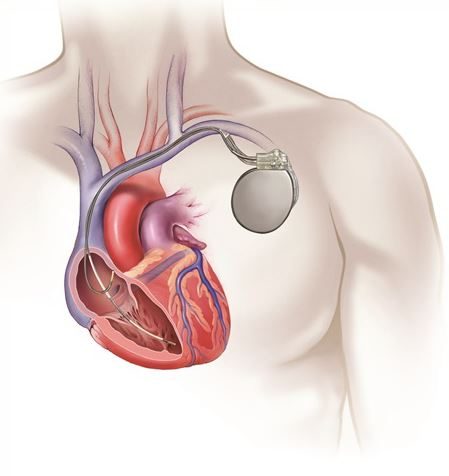
TV-ICD implant procedure
- A transvenous ICD device is typically implanted in the left chest area, near the collarbone.
- Using X-ray imaging, the leads are put through a vein into the heart and across the heart valve.
- Depending on your heart condition, 1 or 2 leads will be placed in the heart. Once the leads are put in place, they are attached to the heart wall.
See below for more information on the similarities and differences between an S-ICD and TV-ICD
| Type | S-ICD | TV-ICD |
|---|---|---|
| Device and lead location | Device: under left arm Lead: under the skin | Device: on upper-left chest Lead: inside the heart |
| Battery life | Approximately 9 years⁴ | Approximately 12 years* |
| Will I have a scar? | Yes - 1 for device on rib cage under left arm 1-2 smaller scars on chest | Yes - 1 for device on upper-left chest |
| Can the device provide pacing if needed? | No - however, if you develop a need for ATP or pacing in the future the S-ICD will be able to be upgraded to provide pacing and/or ATP upon FDA approval of the EMPOWER™ Leadless Pacemaker** | Yes - including ATP and pacemaker capabilities |
| Do I need an EKG screening to be eligible? | Yes | No |
*Battery longevity dependent on device manufacturer, device settings, and amount of pacing required.
** Caution: Investigational Device. Limited by US law to investigational use only. Not available for sale.
See implanted S-ICD and TV-ICD devices
Will the device be visible?
How your implanted device will look can vary based on a number of factors:
- How much body fat you have
- How your body naturally heals
- The size and thickness of your device
- The implant technique (over or under the muscle) Talk to your physician about what you can expect.
S-ICD
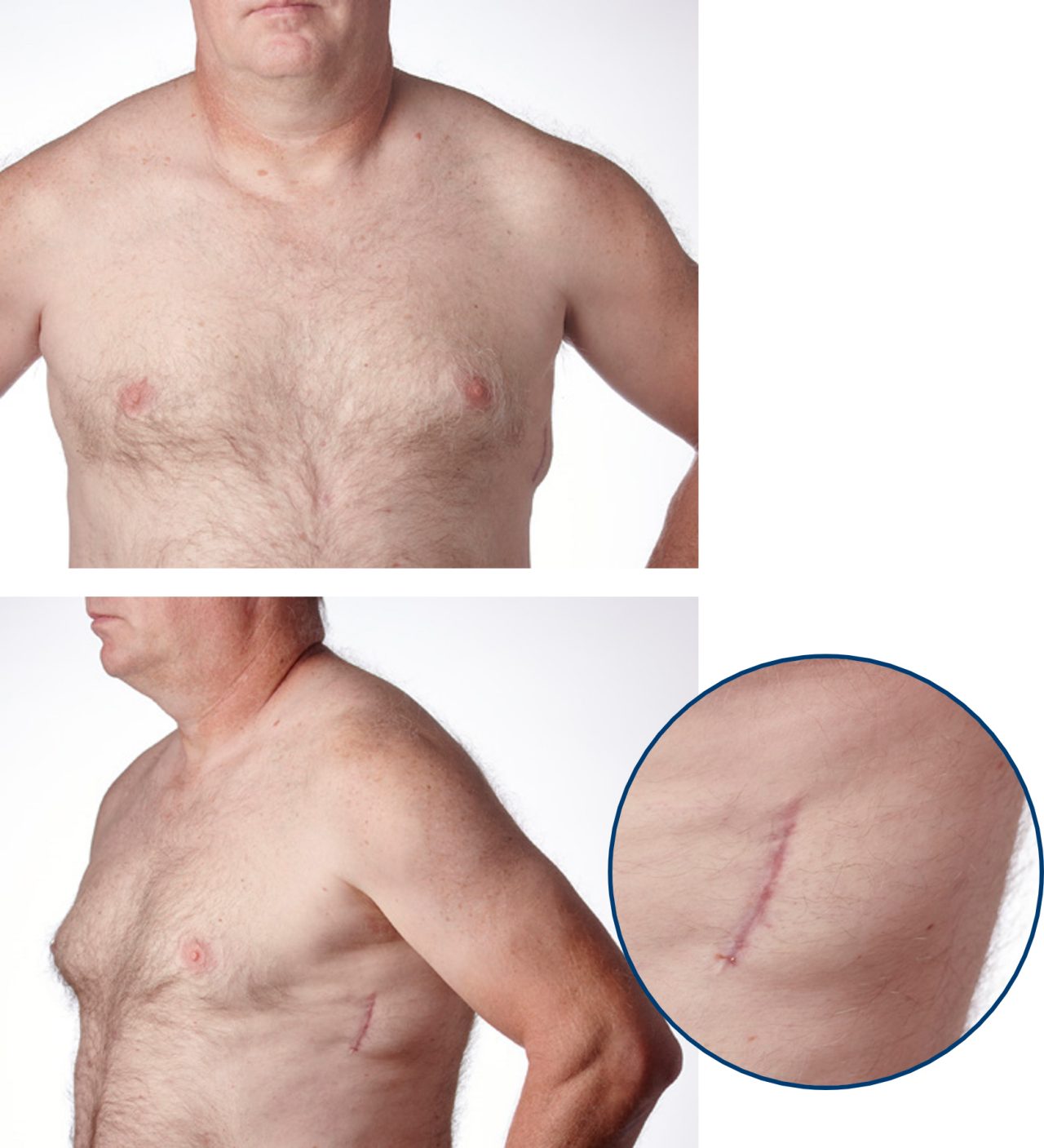
Todd is an EMBLEM™ S-ICD recipient since 2015 and had his first S-ICD replacement 6 weeks prior to these photos.
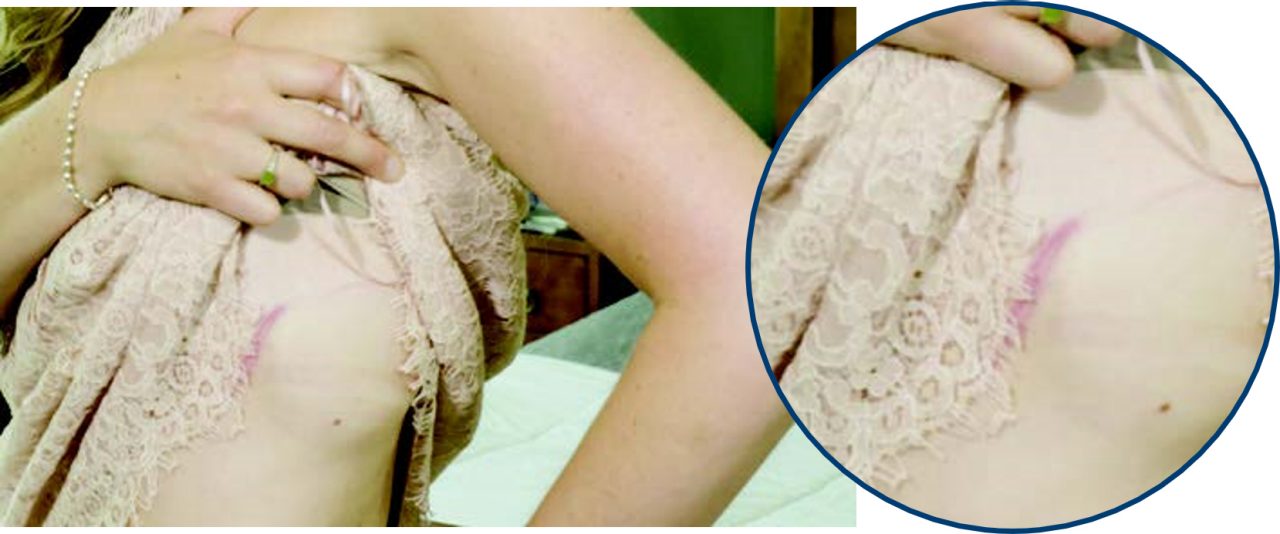
Jan is an EMBLEM™ S-ICD recipient since 2017.
TV-ICD
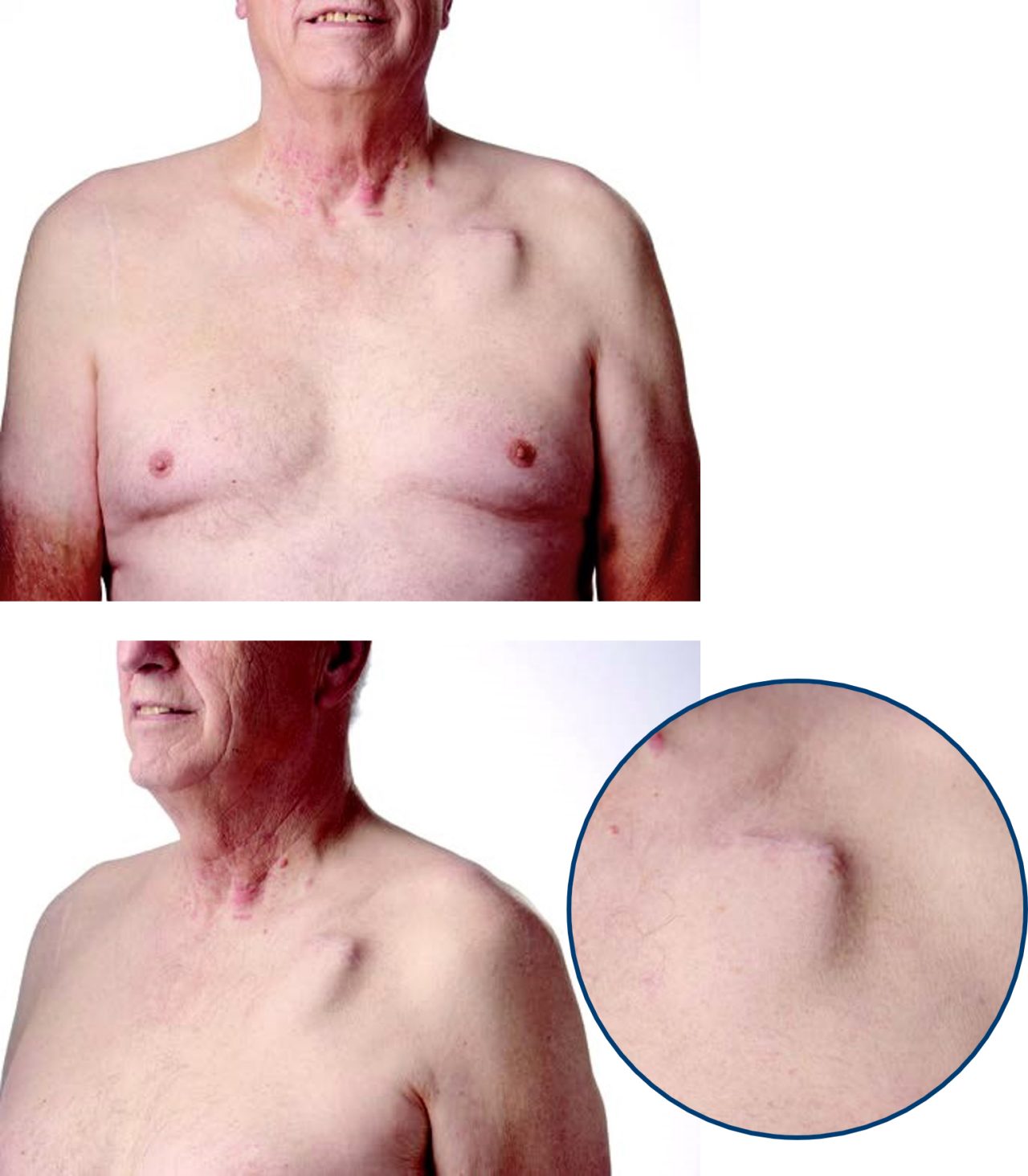
Dennis is a DYNAGEN™ EL (Extended Longevity) ICD recipient since 2018.
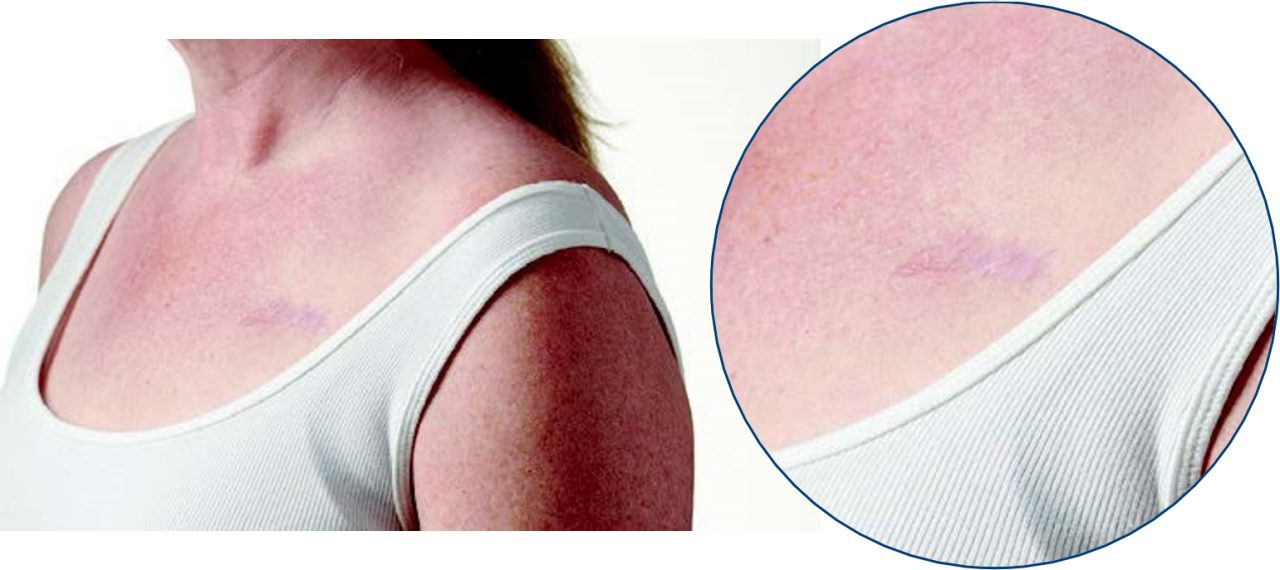
Jen is a TV-ICD recipient since 2019.