The new standard of care for pulmonary embolism treatment
EKOS was the first interventional device cleared for treating pulmonary embolism. Today, it’s trusted by more clinicians than any other PE device.
-
2005
First
patient case - 2008 EKOS receives peripheral vascular indication
- 2008 First DVT data published
- 2021 DVT indication
- 2014 EKOS receives PE indication
- 2021 EKOS+ clearance
- 2022 EKOS+ first patient case
How EKOS works
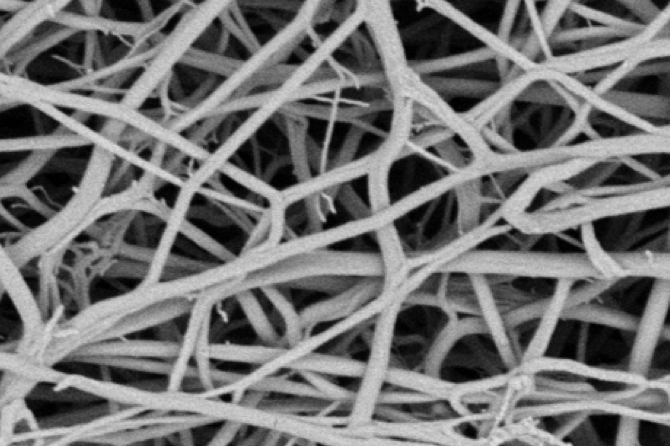
EKOS ultrasound technology unwinds and thins fibrin strands to expose more drug receptor sites; acoustic streaming drives the drug deeper into the clot.
Target thrombus safely
Acoustic pulse
acceleration
Superior clot dissolution
Clinically proven to be safe and reliable with long-term outcomes
- Minimally invasive, 15-minute procedure that is easy to perform
- Low lytic dosing: as low as 8 mg tPA used1
- Prescribed protocol that allows for predictable procedural workflow and proven patient outcomes
Powered by Control Unit 4.0
The EKOS CU4.0 includes an interactive color touchscreen, a built-in battery and separate ports for managing two EKOS devices simultaneously, simplifying bilateral treatment of PE.
1. Tapson V et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism. JACC: Cardiovascular Interventions 2018; 11(14):1401-1410.