REFERENCES:
1. Van der Aa F, Drake MJ, Kasyan GR, et al. The artificial urinary sphincter after a quarter of a century: a critical, systematic review of its use in male non-neurogenic incontinence. Eur Urol. 2013 Apr;63(4):681-9.
2. Lucas MD, Bedretdinova D, Berghmans LC, et al. EAU guidelines on surgical treatment of urinary incontinence. European Association of Urology. March 2015.
3. Data on file with Boston Scientific.
4. Mumm J-N, Klehr B, Rodler S, et al. Five-Year Results of a Prospective Multicenter Trial: AdVance XP for Postprostatectomy- Incontinence in Patients with Favorable Prognostic Factors. Urol Int. 2021;105(5-6):421-427
5. Sandhu JS, Breyer B, Comiter C, et al. Incontinence after Prostate Treatment: AUA/SUFU Guideline. J Urol. 2019 Aug;202(2):369-378.
6. Biardeau X, Aharony S; AUS Consensus Group, et al. Artificial Urinary Sphincter: Report of the 2015 Consensus Conference. Neurourol Urodyn. 2016 Apr;35 Suppl 2:S8-24.
7. Montague DK. Artificial urinary sphincter: long-term results and patient satisfaction. Adv Urol. 2012;2012:835290.
8. James MH, McCammon KA. Artificial urinary sphincter for post-prostatectomy incontinence: a review. Int J Urol. 2014 Jun;21(6):536-43.
9. AMS 800™ Urinary Control System Instructions for Use. American Medical Systems, Inc. 2017 (ERA EL 11)
10. Hüsch T, Kretschmer A, Thomsen F, et al. The AdVance and AdVanceXP male sling in urinary incontinence: is there a difference? World J Urol. 2018 Oct;36(10):1657-1662. (ERA EL 9)
11. Léon P, Chartier-Kastler E, Rouprêt M, et al. Long-term functional outcomes after artificial urinary (ERA EL 10)
12. Boswell TC, Elliott DS, Rangel LJ, et.al. Long-term device survival and quality of life outcomes following artificial urinary sphincter placement. Transl Androl Urol. 2020;9(1):56-61.
13. Cornu JN, Batista Da Costa J, Henry N, et al. Comparative study of AdVance and AdVance XP male slings in a tertiary reference center. Eur Urol. 2014 Feb;65(2):502-4.
14. Rizvi IG, Ravindra P, Pipe M, et al. The AdVance™ male sling: does it stand the test of time? Scand J Urol. 2021 Feb 1:1-6.
15. Joseph JP, Rivera ME, Linder BJ, et al. Evaluating the impact of radiation therapy on patient quality of life following primary artificial urinary sphincter placement. Transl Androl Urol. 2019 Mar;8(Suppl 1):S31-S37.AMS 800™ Artificial Urinary Sphincter
Prior to use, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events.
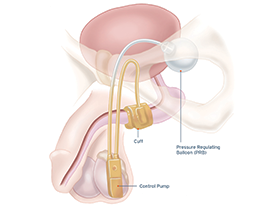
Indications for Use: The AMS 800™ Artificial Urinary Sphincter is used to treat urinary incontinence due to reduced outlet resistance (intrinsic sphincter deficiency) following prostate surgery.
Contraindications: Patients whom the physician determines to be poor surgical candidates, urinary incontinence due to or complicated by an irreversibly obstructed lower urinary tract, have irresolvable detrusor hyperreflexia or bladder instability, or for the AMS 800 with InhibiZone™ Antibiotic Surface Treatment, patients who have a known sensitivity or allergy to rifampin, minocycline or other tetracyclines, or patients with lupus erythematosus because minocycline has been reported to aggravate this condition.
Warnings: Patients with urinary tract infections, diabetes, spinal cord injuries, open sores or regional skin infections may have increased risk of infection associated with a prosthesis. Erosion may be caused by infection, pressure on the tissue, improper cuff sizing, improper balloon selection, tissue damage, and component misplacement. Patients with urge incontinence, overflow incontinence, detrusor hyperreflexia or bladder instability should have these conditions treated and controlled (or resolved) prior to implantation of the device. Surgical, physical, psychological, or mechanical complications, if they occur, may necessitate revision or removal of the prosthesis.
Potential Adverse Events: May include device malfunction/failure leading to additional surgery, device/tissue erosion through urethra/bladder/scrotum, urinary retention, infection, and pain/soreness. MH-545609-AB
AdVance™ XP Male Sling System
Prior to using these devices, please review the Directions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events.
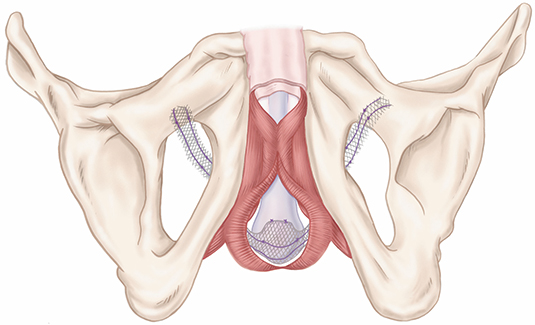
The AdVance™ XP Male Sling System is intended for the placement of a suburethral sling for the treatment of male stress urinary incontinence (SUI). These devices are contraindicated for patients with urinary tract infections, blood coagulation disorders, a compromised immune system or any other condition that would compromise healing, with renal insufficiency, and upper urinary tract relative obstruction. Possible adverse events include, but are not limited to, urinary retention, return to incontinence, pain, erosion, infection, device migration, bleeding, and pelvic organ dysfunction. MH-557013-AA
The person(s) featured in these videos were compensated for their travel and/or time. The people featured in the patient testimonial videos were compensated for their travel and/or time.Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France. All photographs taken by Boston Scientific. All trademarks are the property of their respective owners.
All rights reserved. 2023Copyright © Boston Scientific Corporation or its affiliates.
CE 2797