FAST™ therapy: Paresthesia-free pain relief in minutes
Boston Scientific’s proprietary FAST™ therapy is designed to provide profound paresthesia-free pain relief in minutes. Now patients can experience significant results before they leave your clinic, giving you and your patients confidence in their SCS therapy.
The next era of personalized therapy
Conventional paresthesia-free therapies take time to wash in. However, FAST™ therapy uses precise targeting and proper neural dosing for faster pain relief. FAST™ can also provide:

1. Metzger et al. A novel fast-acting sub-perception spinal cord stimulation therapy enables rapid onset of analgesia in patients with chronic pain,
expert Review of Medical Devices 2021
2. Anitescu M., et al. Clinical impact of a novel fast-acting sub-perception SCS therapy engaging surround inhibition (FAST prospective study), NANS 2023.
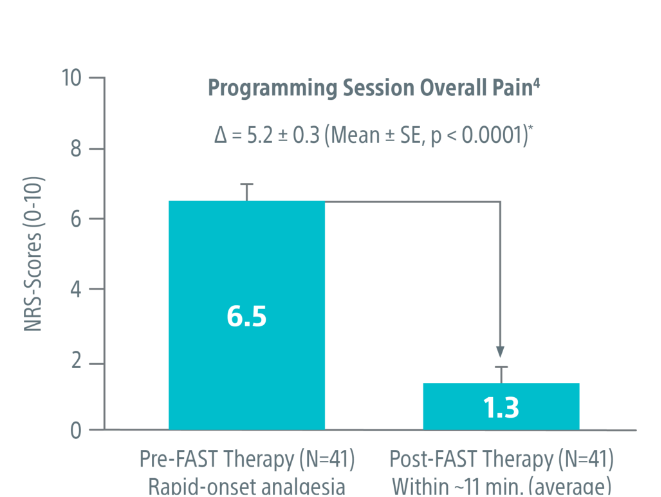
Supported by real-world data
Published real-world data demonstrates the abilities of FAST™ therapy to provide paresthesia-free relief in just minutes. After activation of FAST™ therapy:
- Overall pain was reduced by a mean 5.2 ± 0.3 points versus pre-FAST therapy activation within 11.2 ± 1.9 minutes (N=34)³
- Overall pain was reduced by a mean 7.0 ± 0.4 points (N = 41, p < 0.0001) at six month follow-up.

3. Anitescu M., et al. Clinical impact of a novel fast-acting subperception SCS therapy engaging surround inhibition (FAST prospective study), NANS 2023.
4. FAST MOA computational modeling by Dr. Warren Grill’s lab at Duke University. Gilbert et al. Computational modeling predicts dorsal columns are involved in fast-acting sub-perception spinal cord stimulation (SCS). SFN 2021.
Hear from experts: HCP perspective
Hear from experts: Patient perspective
Hear from patients
Contact and support
The WaveWriter Alpha™ SCS System provides safe access to full-body MRI scans when used with specific components and the patient is exposed to the MRI environment under the defined conditions in the ImageReady™ MRI Full Body Guidelines for WaveWriter Alpha and WaveWriter Alpha Prime Spinal Cord Stimulator System. The Bluetooth® word mark and logos are registered trademarks owned by the Bluetooth SIG, Inc. and any use of such marks by Boston Scientific Neuromodulation Corporation is under license. Product available in the European Economic Area (EEA) only. Please check availability with your local sales representative or customer service.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material is not intended for use in France.
















