SYNERGY™
Everolimus-Eluting Platinum Chromium Coronary Stent System
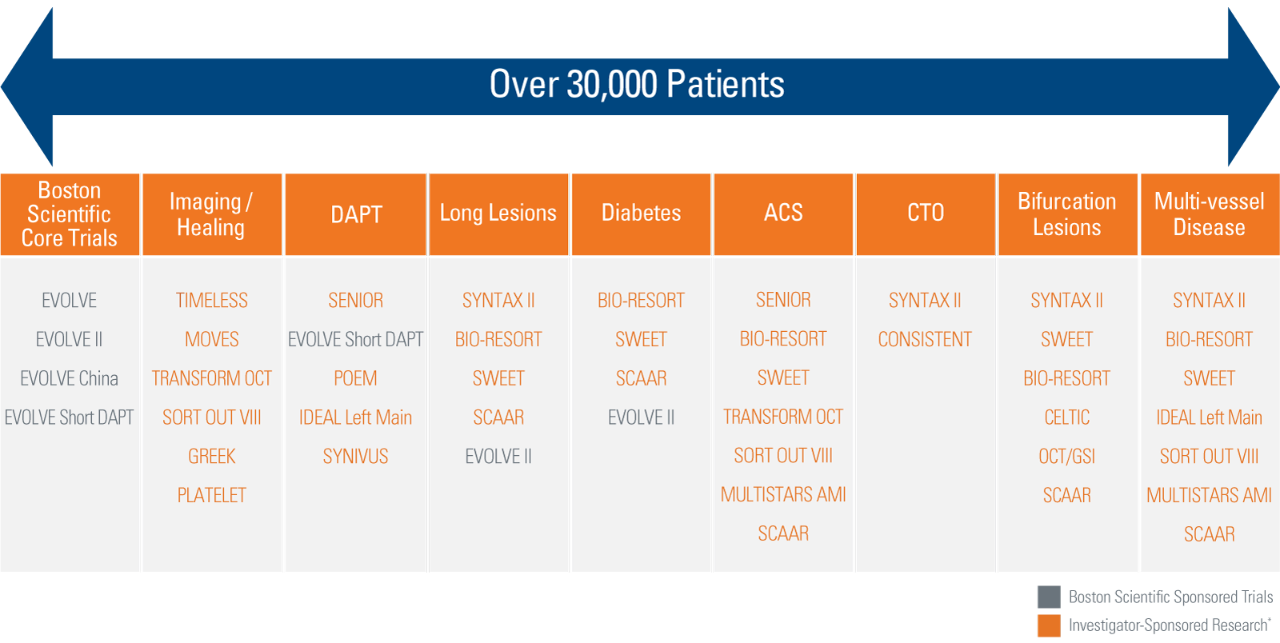
SYNERGY BP Stent Clinical Program and Investigator-Sponsored Research*
The safety and efficacy of the SYNERGY Stent has not been established in CTOs
* Boston Scientific is not responsible for the collection, analysis or reporting of the investigator-sponsored research output which is the sole responsibility of the investigators. Boston Scientific’s involvement in investigator-sponsored research is limited to providing financial support for research that advances medical and scientific knowledge about our products.
Boston Scientific Clinical Trial Program
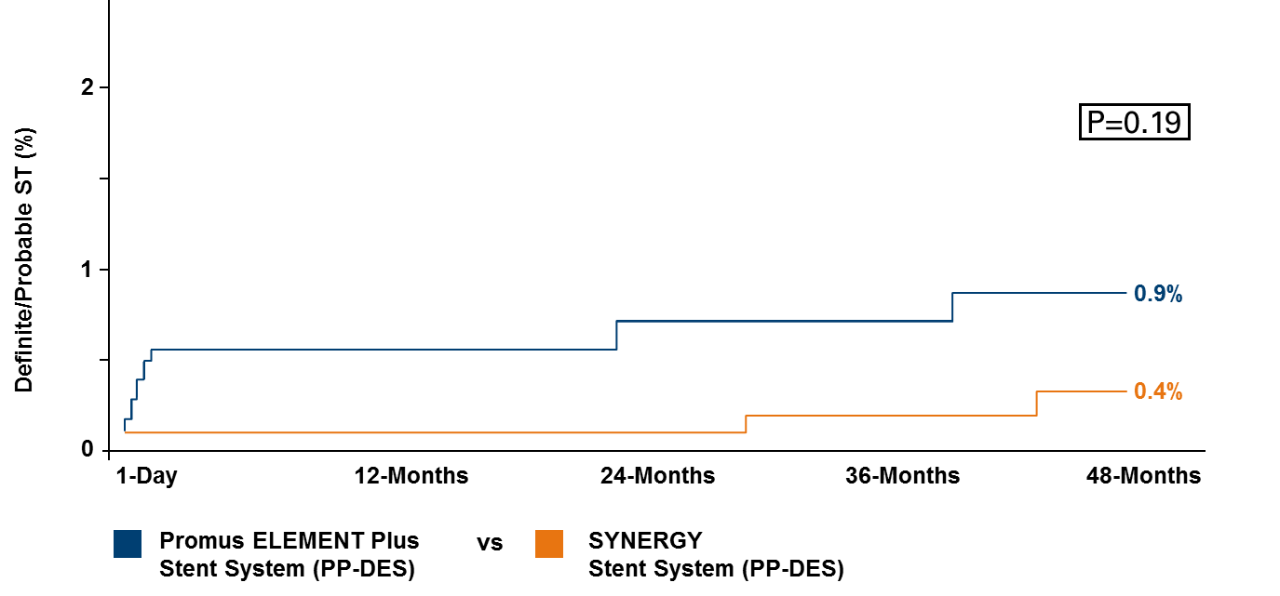
† CEC confirmed MI/TLR/ST Day 901 in the SYNERGY arm 37 y/o male patient had 1,1,0 distal RCA/PDA bifurcation lesion, and a second lesion in the mid-LAD treated during the index procedure (patient was discharged on DAPT [clopidogrel]. On day 840, patient had TLR of 75% in-stent restenosis of the distal RCA/PDA lesion performed with drug coated balloon (patient was discharged on DAPT [prasugrel]). On day 901, patient developed severe chest pain, ST elevation and marked elevation of cardiac enzymes. Found to have ST of RCA/PDA lesion, which was successfully treated with another stent. ITT; Patients who did not receive a study stent were censored at 1 year; KM Event Rate; log-rank P values.
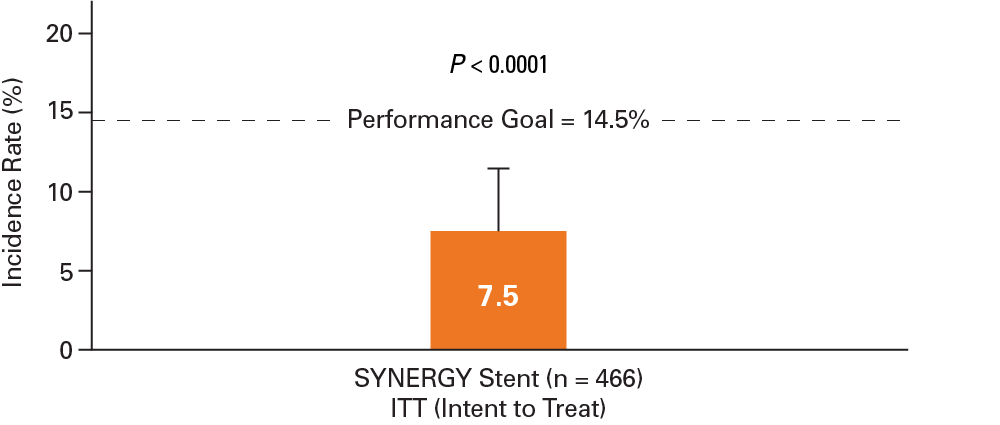
P-value from the one-sided Clopper-Pearson test is <0.025, the 12-month TLF rate from SYNERGY is concluded to be less than the performance goal (14.5%)
One-sided 97.5% Clopper Pearon Upper Confidence Bound (UCB) Target lesion failure (TLF) defined as: Cardiac death, or MI related to the target vessel (based on CK-MB >3x URL), or Ischemia-driven target lesion revascularization
The EVOLVE first human use trial demonstrated outstanding long-term safety and performance with the SYNERGY BP Stent.
EVOLVE Trial 5-Year Results3