SYNERGY™
Everolimus-Eluting Platinum Chromium Coronary Stent System
EVOLVE Short DAPT Trial*
The SYNERGY BP Stent can be safely used in conjunction with shortened DAPT in patients at high risk for bleeding (HBR), based on the results from the EVOLVE Short DAPT Trial. This trial data in conjunction with the HBR indication, provides important evidence to inform decisions about DAPT duration for this patient subset.
“These data better inform physicians as to how best to tailor the recommended duration of DAPT to the bleeding risk of the patients they treat.”
- Dr. Ajay Kirtane
Director of Cardiac Catheterization Laboratories at Columbia University Irving Medical Center/New York-Presbyterian Hospital and principal investigator of the EVOLVE Short DAPT trial
As quoted in Bioworld.com
POEM Trial
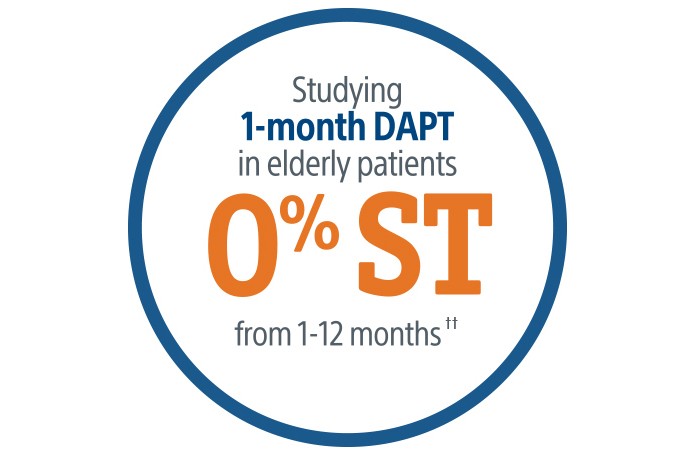
The POEM trial evaluated the safety of SYNERGY with 1-month DAPT in HBR patients. At 1 year, SYNERGY with 1-month DAPT demonstrated a low rate of ischemic and bleeding events in an all-comers HBR patient population.†‡²
Average # of HBR criteria met per patient: 1.7 |
Additional Short DAPT Data
Boston Scientific continues to support short DAPT trials with over 5,000 patients to study the SYNERGY BP Stent. We know every patient needs individualized care. That is why we are supporting short DAPT studies in a variety of patient populations and DAPT durations. The SYNERGY BP Stent has shown exceptionally low ST rates across short DAPT trials:§
Hear more about the SYNERGY design & DAPT from Drs. Aloke Finn and Robert Yeh at TCT 2019