Solyx™
Single-Incision Sling System
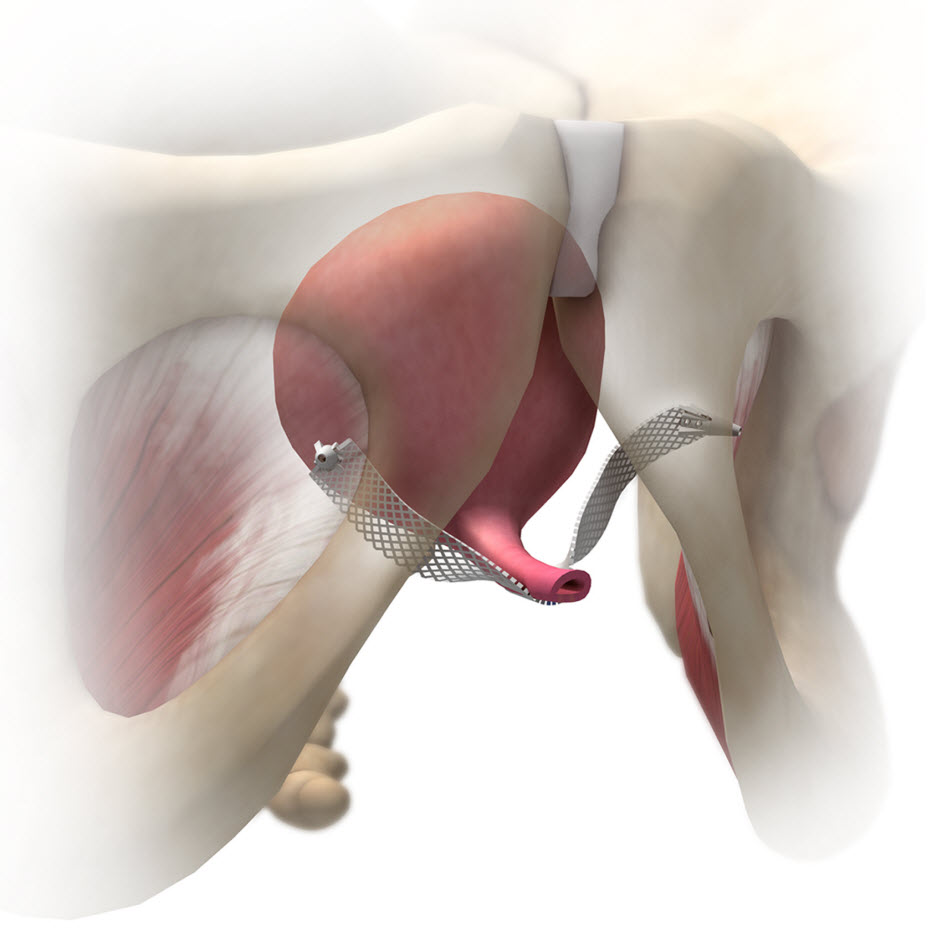
Single incision sling system designed to offer a procedure with fewer steps and reduced dissection. The mesh assembly is designed to be placed away from critical structures, such as the obturator bundle, and the carrier snap-fit on the delivery device tip is designed to facilitate control during placement. Available with Advantage™ clear mesh or Advantage Blue mesh.
Key Resources
Product Details
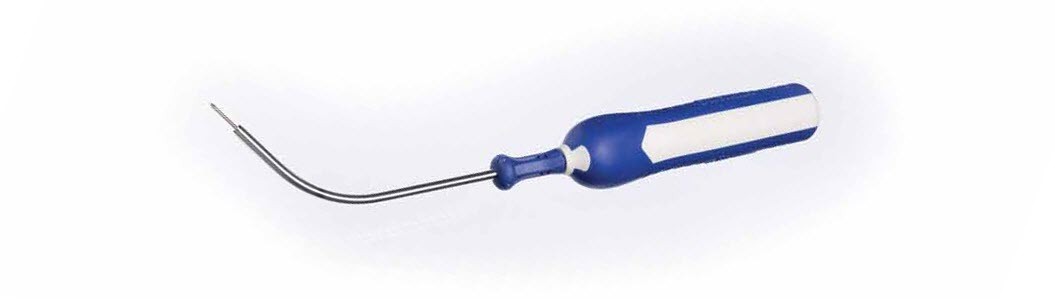
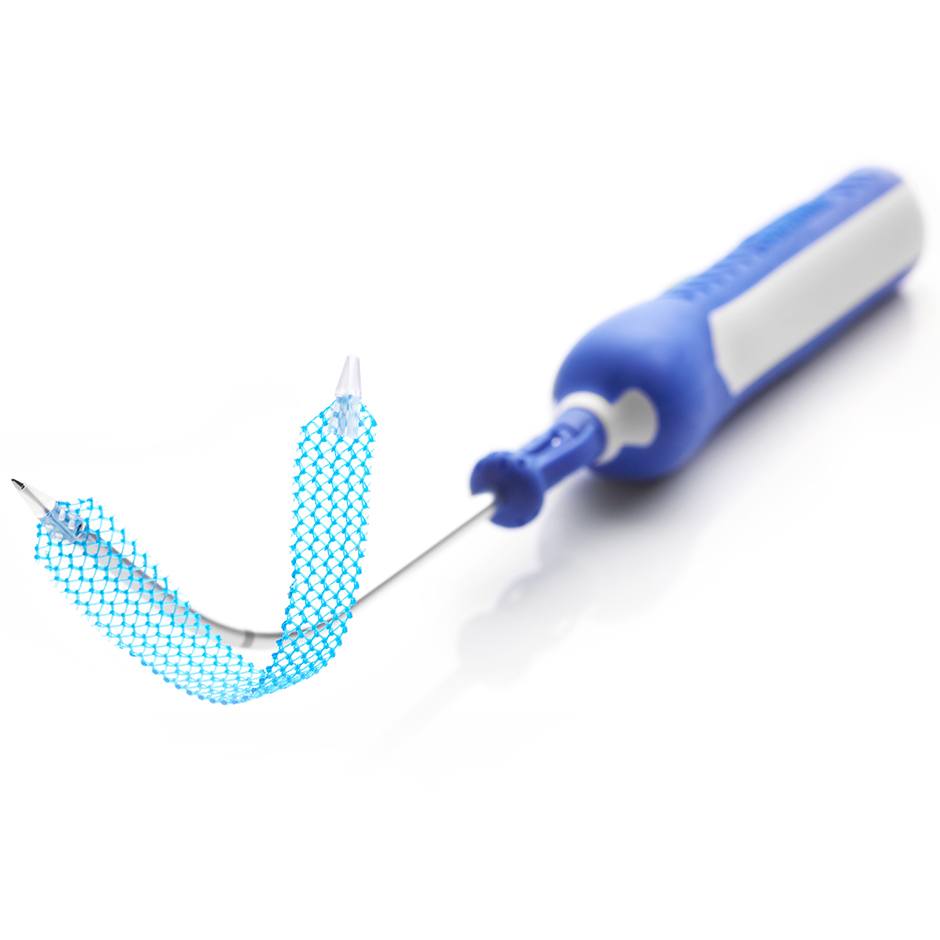
Mesh Carrier and Assembly
- The barb design is intended to track smoothly through tissue
- Snap-fit delivery device is designed to help prevent premature carrier slip-off
- Mesh length is 9cm
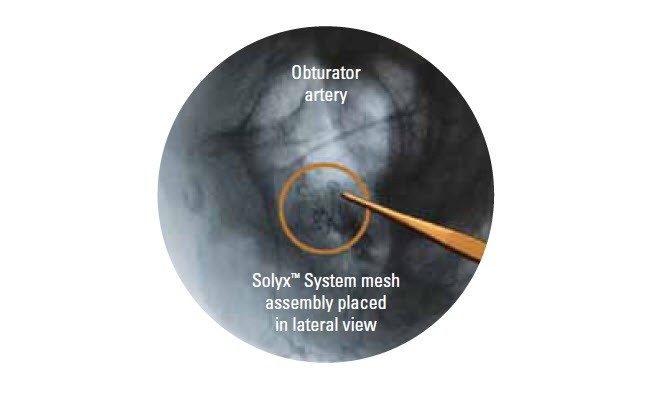
- Mesh assembly placement is designed to be away from critical structures, such as the obturator bundle
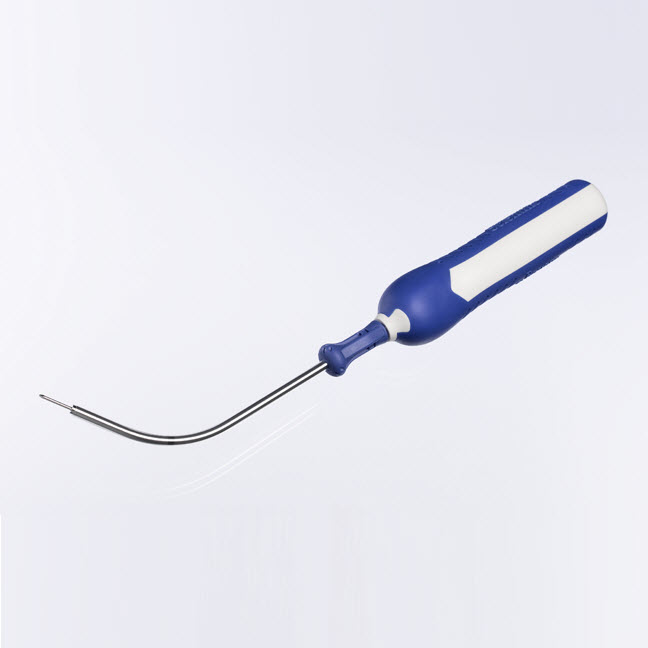
Delivery Device
- Designed to seat carrier where placed
- Ergonomic handle design
- Mid-line marker designed to facilitate guidance for accurate placement
Ability to Tighten
- Sling is tensioned by delivery device advancement
- Carrier snap-fit on delivery device tip designed to facilitate control during placement
- Note: Once the carrier is deposited in the tissue, it is not designed to be reconnected onto the shaft tip for additional tension/adjustment.
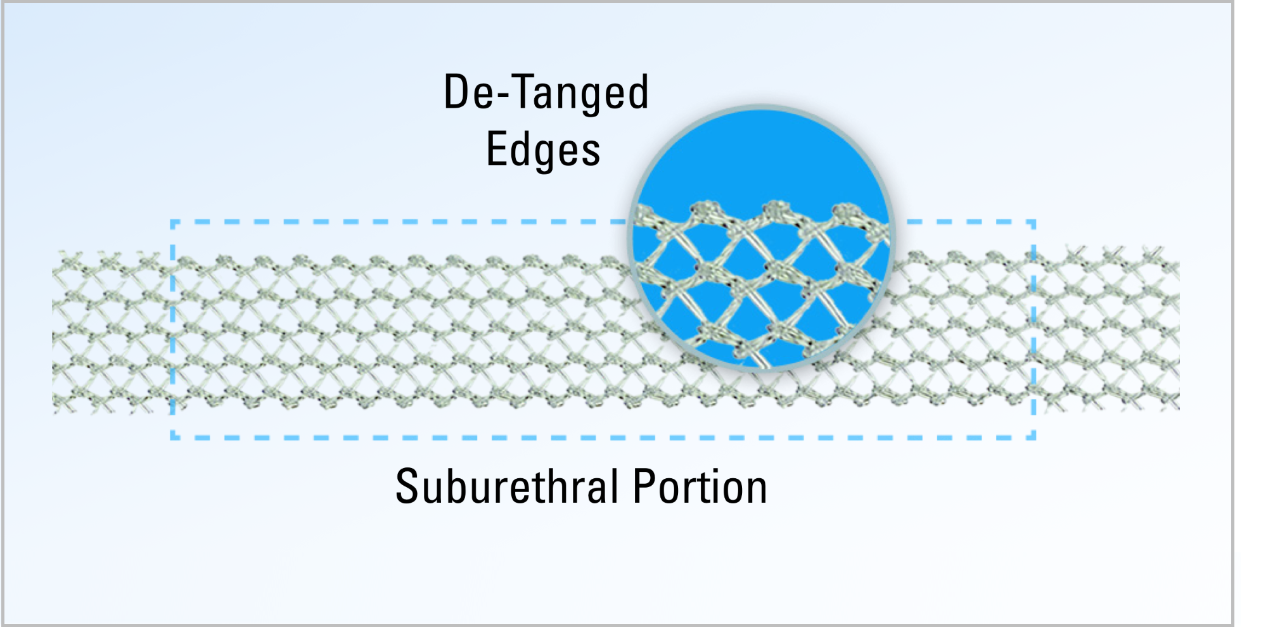
De-Tanged Polypropylene Material
- A de-tanged suburethral portion designed to maintain its integrity during tensioning and potentially reduce irritation to the urethral wall
Testimonials
If you treat patients with stress urinary incontinence, you know the journey can be long and life changing. Often, women wait years before they make their first appointment, but when they do seek treatment their lives can be improved.
Explore the journey from initiating the conversation to seeing lives impacted by the Solyx™ Single-Incision Sling in this short video series featuring Dr. Kevin Benson, a urogynecologist from Sanford Health, and two patients, AJ and Chandra.
Episode 1: Beginning the conversation
Episode 2: Evaluating for treatment
Episode 3: Back to enjoying life
Ordering Information
| Order Number | UPN | Description | Quantity |
|---|---|---|---|
| 850700 | M0068507000 | Solyx SIS System | 1 Delivery Device and 1 Mesh Assembly |
| 850701 | M0068507010 | Solyx Blue Single Incision Sling System | 1 Delivery Device and 1 Mesh Assembly |
Each System includes One (1) Delivery Device and One (1) Mesh Assembly
Get in touch with our team
Whether you’re looking to trial a Solyx Single-Incision Sling System or have general questions about the products, please fill out the fields below and a member of our team will contact you.