The Difference is in the Data
36-MONTH DATA FROM SOLYX SINGLE INCISION SLING SYSTEM 522 STUDY
A Prospective Parallel Cohort, Multi-center Study of the Solyx™ Single Incision Sling System vs. the Obtryx™ II Transobturator Mid-Urethral Sling System for the Treatment of Women with Stress Urinary Incontinence: 3-Year Results.
Solyx Single Incision Sling System 522 post-market clinical study

Primary Endpoint Results
| Treatment Success | Solyx (n=104) | Obtryx II (n=108) |
|---|---|---|
| Composite | 90.4% | 88.9% |
| Objective | 94.2% | 91.7% |
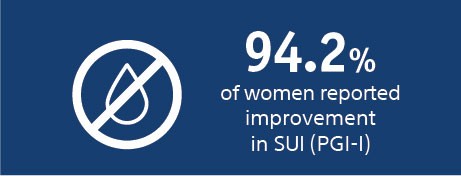
| Subjective | 94.2% | 94.4% |
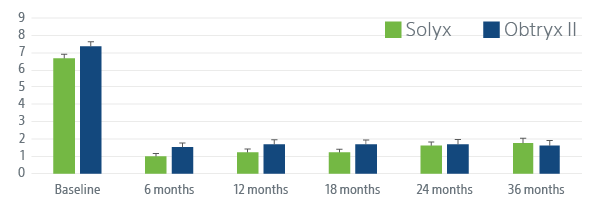
SOLYX IMPROVED SEXUAL FUNCTION
Following the procedure women reported better sexual function at each follow up visit.
Pelvic Impact Sexual Questionnaire (PISQ-12)
| Treatment | Propensity Adjusted Treatment Difference | |||
| Study Visit | SIS† | TMUS† | Estimate [95% CI] | p-value |
| Baseline | 33.3 ± 7.1(113) (13.0,35.0,47.0) | 33.7 ± 6.5(103) (15.0,35.0,45.0) | -0.9 [-3.0 , 1.3] | 0.425 |
| 6 months | 38.9 ± 4.7(97) (22.0,40.0,46.0) | 39.4 ± 4.8(93) (25.0,40.0,47.0) | -0.5 [-2.2 , 1.3] | 0.603 |
| 12 months | 39.0 ± 4.6(88) (25.0,40.0,46.0) | 39.8 ± 5.2(90) (16.0,41.0,48.0) | -0.9 [-2.8 , 0.9] | 0.318 |
| 18 months | 38.5 ± 5.6(87) 15.0,39.0,46.0) | 40.4 ± 5.3(90) (25.0,42.0,47.0) | -1.7 [-3.7 , 0.3] | 0.093 |
| 24 months | 38.5 ± 4.8(79) (24.0,40.0,46.0) | 39.6 ± 5.3(82) (24.0,41.0,48.0) | -0.9 [-3.1 , 1.3] | 0.421 |
| 36 months | 37.9 ± 5.6(80) (22.0,39.0,45.0) | 40.1 ± 5.2(77) (18.0,41.0,48.0) | -2.5 [-4.7 , -0.2] | 0.031 |
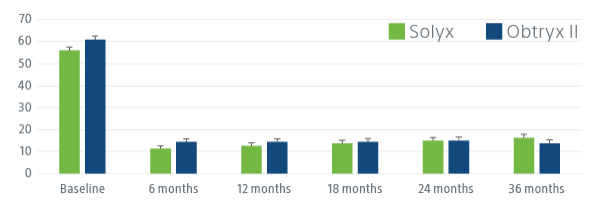
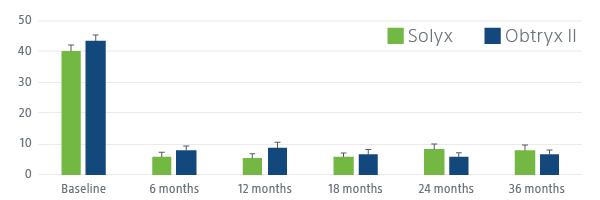
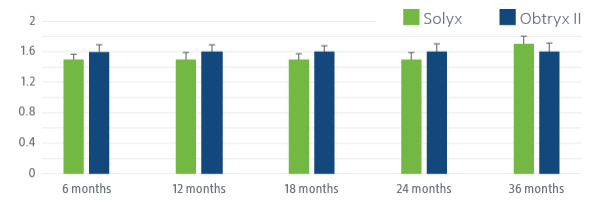
SOLYX DEMONSTRATED STATISTICALLY SIGNIFICANT PATIENT IMPROVEMENT
Solyx improved women’s symptom bother, symptom severity and quality of life.
Following SIS (Solyx) and TMUS (Obtryx II) patients have significant improvement in patient-reported outcomes, including UDI-6, ISI and UIQ-7 at 36 months, indicating disease-specific QoL improvement. Patients have a more positive impression of change in stress UI symptoms at each follow-up visit, indicating generic QoL improvement.
Adverse Events
| Adverse Events | Solyx Intent-to-Treat Subjects (N=141) | Obtryx II Intent-to-Treat Subjects (N=140) | |||
| Events | Proportion of Subjects with ≥1 Events | Events | Proportion of Subjects with ≥1 Events | p-value | |
| Mesh exposurea | 4 | 2.8% | 6 | 4.3% | 0.541 |
| Dyspareuniab | 1 | 0.7% | 0 | 0.0% | 1.000 |
| Mesh Erosionc | 0 | 0.0% | 1 | 0.7% | 0.498 |
| Pelvic Paind | 1 | 0.7% | 0 | 0.0% | 1.000 |
| Urinary Retentione | 4 | 2.8% | 6 | 4.3% | 0.541 |
b. Dyspareunia defined as any new onset pain associated with sexual activity that was not present during sexual activity preoperatively; or any worsening pain associated with sexual activity compared to preoperative state.
c. Mesh erosion defined as perforation of mesh into hollow organ or viscus.
d. Pelvic pain defined as any pain associated with worsening bother compared to preop occurring in the lower abdomen or genital area beyond 12 weeks post-operatively (excluding neuromuscular pain and dyspareunia).
e. Urinary retention defined as inability to completely empty the bladder.

Solyx demonstrated comparable outcomes to Obtryx II showing its efficacy, improved patient quality of life and improvement in sexual function over the course of 3 years.
Comparison of Two Single Incision Slings on the Vagina in an Ovine Model
Published as a full manuscript in the peer-reviewed American Journal of Obstetrics and Gynecology, this study demonstrated favorable performance in host biochemical and mechanical response of Boston Scientific’s Solyx Single Incision Sling (SIS-A) when compared with Coloplast’s Altis (SIS-B).
Background & Objective
For prolapse repair, meshes with higher porosity and lower structural stiffness have been associated with improved outcomes, but research has not explored the impact of these mesh characteristics for SIS. The study was conducted to compare the host response of two currently available SIS in an animal model — the lower-stiffness, higher-porosity Solyx SIS and the higher-stiffness, lower-porosity Altis SIS.
Results
| Solyx (SIS-A) | Altis (SIS-B) | ||
|---|---|---|---|
| Buckled in only 1 specimen (8%, n=1/13, P=0.004) | Consistently buckled (73%, n=8/11) | ||
| Optimal inter-fiber distance and pore size (>1 mm) | Sub-optimal inter-fiber distance and pore size (<1 mm) | ||
| Demonstrated improved tissue integration (73% vs. 27%, P<0.05) | Higher amounts of both collagen I and III, suggesting greater microinjury to surrounding tissue |
| Solyx (SIS-A) | |
|---|---|
| Buckled in only 1 specimen (8%, n=1/13, P=0.004) | |
| Optimal inter-fiber distance and pore size (>1 mm) | |
| Demonstrated improved tissue integration (73% vs. 27%, P<0.05) | |
| Altis (SIS-B) | |
| Consistently buckled (73%, n=8/11) | |
| Sub-optimal inter-fiber distance and pore size (<1 mm) | |
| Higher amounts of both collagen I and III, suggesting greater microinjury to surrounding tissue |
Additional Clinical Research
Boston Scientific actively partners with leading researchers in two additional large-scale clinical projects.
Our Ongoing Commitment to Clinical Excellence
Boston Scientific is committed to supporting evidence-based medicine through sponsoring and funding pelvic floor research worldwide.
3500+women treated with our products in clinical studies around the world |
25+years of physician-driven, customer-centric innovation |
100+articles and abstracts |
Numerousongoing investigator-sponsored research (ISR) studies |
$30 million+in post-market 522 studies and publications |
2additional large-scale clinical projects |
3500+women treated with our products in clinical studies around the world |
150+physicians collaborating on research |
100+clinical studies / publications |
14ongoing investigator-sponsored research (ISR) studies |
$32 millionin post-market 522 studies and publications |
2additional large-scale clinical projects |