Thank you for your interest in the OPTION Trial. This trial is active and has reached full enrollment.
Please find information on patient inclusion criteria for a currently enrolling WATCHMAN FLX™ LAAC vs NOAC stroke risk reduction trial.
The OPTION Clinical Trial
What is the OPTION Clinical Trial?
The OPTION Clinical Trial is a randomized controlled trial comparing the safety and effectiveness of left atrial appendage closure (LAAC) to oral anticoagulation (OAC) therapy for stroke protection in post-ablation patients with atrial fibrillation.
This study involves an investigational device called the WATCHMAN FLX™*.
Study Overview
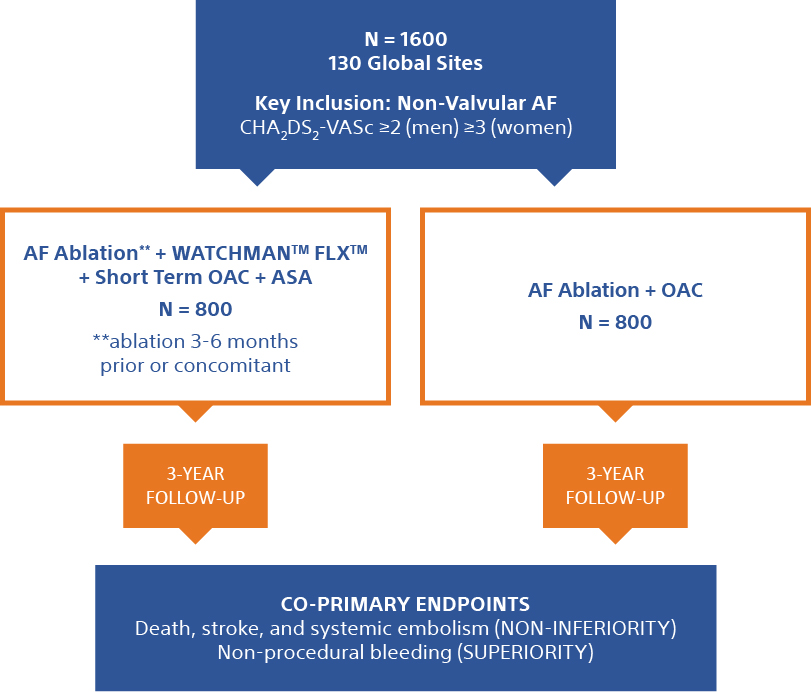
WATCHMAN FLX is an FDA approved device being studied for an expanded indication as a first line therapy vs NOAC for NVAF patients post-ablation. The use of WATCHMAN or WATCHMAN FLX as a first-line therapy for stroke risk reduction in NVAF patients post-ablation is considered investigational.
Caution: Investigational Device. Limited by US law to investigational use only. Not available for sale.
CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the complete “Instructions for Use” for more information on Indications, Contraindications, Warnings, Precautions, Adverse Events, and Operator’s Instructions.
INTENDED USE/INDICATIONS FOR USE
The WATCHMAN FLX Device is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with non-valvular atrial fibrillation who:
• Are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores and are recommended for anticoagulation therapy;
• Are deemed by their physicians to be suitable for anticoagulation therapy; and
• Have an appropriate rationale to seek a non-pharmacologic alternative to anticoagulation therapy, taking into account the safety and effectiveness of the device compared to anticoagulation therapy.
CONTRAINDICATIONS
Do not use the WATCHMAN FLX Device if:
• Intracardiac thrombus is present.
• An atrial septal defect repair or closure device or a patent foramen ovale repair or closure device is present.
• The LAA anatomy will not accommodate a Closure Device (see Table 45 of the eIFU).
• The patient has a known hypersensitivity to any portion of the device material or the individual components (see Device Description section of the eIFU) such that the use of the WATCHMAN FLX Device is contraindicated.
• Any of the customary contraindications for other percutaneous catheterization procedure (e.g., patient size too small to accommodate TEE probe or required catheters) or conditions (e.g., active infection, bleeding disorder) are present.
• There are contraindications to the use of anticoagulation therapy, aspirin, or P2Y12 inhibitor.
WARNINGS
Implantation of the WATCHMAN FLX Device should only be performed by interventional cardiologists and/or electrophysiologists who are trained in percutaneous and transseptal procedures and who have completed the WATCHMAN FLX Physician Training program.
• This device has not been studied in pregnant or breastfeeding women. Careful consideration should be given to use of the Closure Device in pregnant and/ or breastfeeding women due to the risk of significant exposure to x-rays and the use of anticoagulation medication.
• Device selection should be based on accurate LAA measurements obtained using echocardiographic imaging guidance in multiple views (TEE recommended in multiple angles [e.g., 0°, 45°, 90°, 135°]) to avoid improper Closure Device sizing.
• Do not release (i.e., unscrew) the WATCHMAN FLX Device from the core wire unless all release criteria are satisfied to avoid suboptimal results.
• Potential for Closure Device embolization exists with cardioversion < 30 days following Closure Device implantation; verify Closure Device position after cardioversion during this period.
• Appropriate post-procedure drug therapy should be followed. See Post-Procedure Information section (of the eIFU) for further detail.
PRECAUTIONS
• The safety and effectiveness (and benefit-risk profile) of the WATCHMAN FLX Device has not been established in patients for whom long-term anticoagulation is determined to be contraindicated.
• The LAA is a thin-walled structure. Use caution when accessing the LAA, and deploying, recapturing, and repositioning the Closure Device.
• Use caution when introducing a WATCHMAN Access System to prevent damage to cardiac structures.
• Use caution when introducing the Delivery System to prevent damage to cardiac structures.
• To prevent damage to the Delivery Catheter or Closure Device, do not allow the WATCHMAN FLX Device to protrude beyond the distal tip of the Delivery Catheter when inserting the Delivery System into the Access Sheath.
• If using a power injector, the maximum pressure should not exceed 100 psi.
PATIENT SELECTION FOR TREATMENT
In considering the use of the WATCHMAN FLX Device, the rationale for seeking an alternative to long-term anticoagulation therapy and the safety and effectiveness of the device compared to anticoagulation should be taken into account.
• The presence of indication(s) for long-term anticoagulation therapy, other than non-valvular atrial fibrillation (e.g. mechanical heart valve, hypercoagulable states, recurrent deep venous thrombosis). Details regarding the indications, contraindications, warnings, and precautions for oral anticoagulants approved for patients with non-valvular atrial fibrillation are provided in their respective Instructions for Use.
Of note:
• The safety and effectiveness (and benefit-risk profile) of the WATCHMAN FLX Device has not been established in patients for whom long-term anticoagulation is determined to be contraindicated. Factors that need to be considered for the WATCHMAN FLX Device and implantation procedure include the following:
• Overall medical status, including conditions which might preclude the safety of a percutaneous, transcatheter procedure.
• Suitability for percutaneous, transseptal procedures, including considerations of:
– Cardiac anatomy relating to the LAA size and shape.
– Vascular access anatomy (e.g., femoral vein size, thrombus, or tortuosity).
– Ability of the patient to tolerate general or local anesthesia.
– Ability of the patient to undergo required imaging.
• Ability to comply with the recommended post-WATCHMAN FLX Device implant pharmacologic regimen (see Post-Procedure Information section) especially for patients at high risk for bleeding.
ADVERSE EVENTS
Potential adverse events (in alphabetical order) which may be associated with the use of a left atrial appendage closure device or implantation procedure include but are not limited to: • Air embolism • Airway trauma • Allergic reaction to the contrast media, anesthetic, WATCHMAN Implant material, or medications • Altered mental status • Anemia requiring transfusion • Anesthesia risks • Angina • Anoxic encephalopathy • Arrhythmias • Atrial septal defect • Bruising, hematoma, or seroma near the catheter insertion site • Cardiac perforation • Chest pain/discomfort • Confusion post procedure • Congestive heart failure • Contrast related nephropathy • Cranial bleed • Death • Decreased hemoglobin • Deep vein thrombosis • Device embolism • Device fracture • Device thrombosis • Edema • Embolism • Excessive bleeding • Fever • Fistula • Groin pain • Groin puncture bleed • Hematuria • Hemoptysis • Hypotension • Hypoxia • Improper wound healing • Inability to reposition, recapture, or retrieve the device • Infection/pneumonia • Interatrial septum thrombus • Intratracheal bleeding • Major bleeding requiring transfusion • Misplacement of the device/improper seal of the appendage/movement of device from appendage wall • Myocardial erosion • Nausea • Oral bleeding • Pericardial effusion/tamponade • Pleural effusion • Prolonged bleeding from a laceration • Pseudoaneurysm • Pulmonary edema • Renal failure • Respiratory insufficiency/failure • Stroke – Hemorrhagic • Stroke – Ischemic • Surgical removal of the device • TEE complications (e.g., throat pain, bleeding, esophageal trauma) • Thrombocytopenia • Thrombosis • Transient ischemic attack (TIA) • Valvular or vascular damage • Vasovagal reactions
There may be other potential adverse events that are unforeseen at this time.
92574167 A.1
