Thank you for your interest in the OPTION Trial. This trial is active and has reached full enrollment.
OPTION - AFib, Ablation, and Stroke - Boston Scientific
What Is AFib?
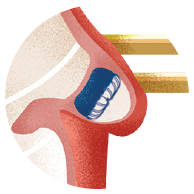
Atrial Fibrillation (AFib) is a heart condition in which the upper chambers of your heart quiver. This condition can cause blood clots to form in an area of your heart called the left atrial appendage (LAA). If a blood clot forms here, it can travel through an artery to the brain and cause a stroke.
People with AFib have a five-times greater risk for stroke than people with normal heart rhythms.1
Managing AFib and Stroke Risk
While completely curing AFib may not be possible, medications and/or treatments such as an AFib ablation, a procedure to burn or freeze the tissue around the veins in the upper left chamber of your heart, can help decrease the burden of AFib. However, even after an ablation, you are still at risk of having a stroke.
Blood thinners or Left Atrial Appendage Closure (LAAC) implants like WATCHMAN address the need for stroke reduction in AFib patients.
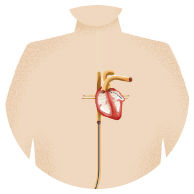
WATCHMAN is a one-time procedure that does not require open heart surgery and may reduce stroke risk for a lifetime. The device is about the size of a quarter, and eliminates the need for long-term blood thinner use. It is implanted into the left atrial appendage of your heart to permanently close off this small pouch and keep harmful blood clots from escaping and causing a stroke.
The Implant Procedure


Important Safety Information
The WATCHMAN and WATCHMAN FLX Devices are permanent implants designed to close the left atrial appendage in the heart in an effort to reduce the risk of stroke.
With all medical procedures there are risks associated with the implant procedure and the use of the device. The risks include but are not limited to accidental heart puncture, air embolism, allergic reaction, anemia, anesthesia risks, arrhythmias, AV (Arteriovenous) fistula, bleeding or throat pain from the TEE (Trans Esophageal Echo) probe, blood clot or air bubbles in the lungs or other organs, bruising at the catheter insertion site, clot formation on the device, cranial bleed, excessive bleeding, gastrointestinal bleeding, groin puncture bleed, hypotension, infection/pneumonia, pneumothorax, pulmonary edema, pulmonary vein obstruction, renal failure, stroke, thrombosis and transient ischemic attack. In rare cases death can occur.
Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of the device.

