CHAMPION-AF Clinical Trial
Why CHAMPION-AF?
The CHAMPION-AF trial evaluates LAAC versus NOAC in a broad NVAF patient population to establish WATCHMAN FLXTM as a first-line stroke risk-reduction therapy.
LAAC with WATCHMANTM or WATCHMAN FLX is currently used for patients who can tolerate short-term oral anticoagulation (OAC) therapy, but are considered poor candidates for long-term OAC.
However, there is a large population of low to moderate bleed risk patients* who can tolerate long term OAC, but would benefit from a one time, FDA-approved device alternative that offers a lifetime of safe and effective stroke risk reduction.
Trial Design and Primary Endpoints
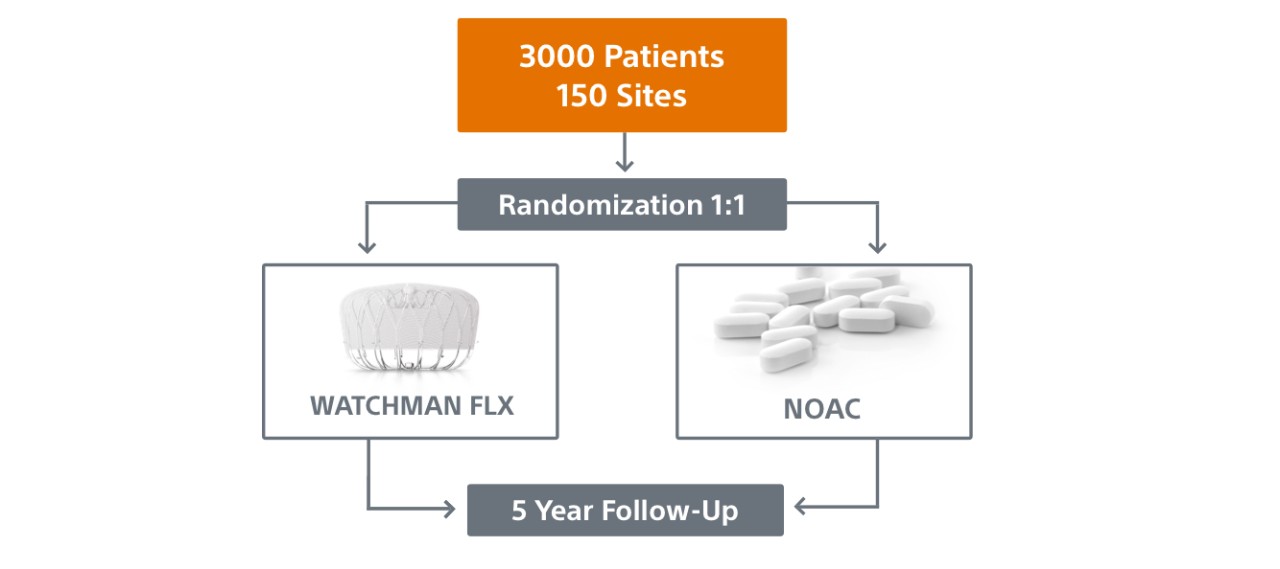
Primary Endpoints:
- WATCHMAN FLX is non-inferior for the occurrence of stroke (including ischemic and/or hemorrhagic), cardiovascular (CV) death (including hemorrhagic and/or unexplained death), and systemic embolism at 36 months.
- WATCHMAN FLX is superior for non-procedural bleeding (ISTH major bleeding and clinically relevant non-major bleeding) at 36 months.
- WATCHMAN FLX is non-inferior for the occurrence of ischemic stroke and systemic embolism at 60 months.
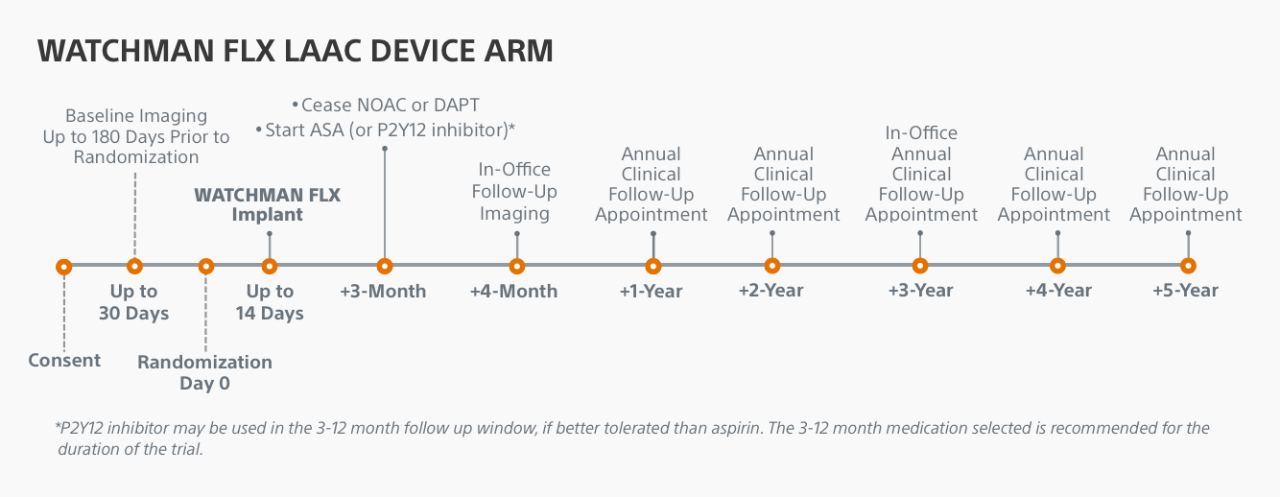
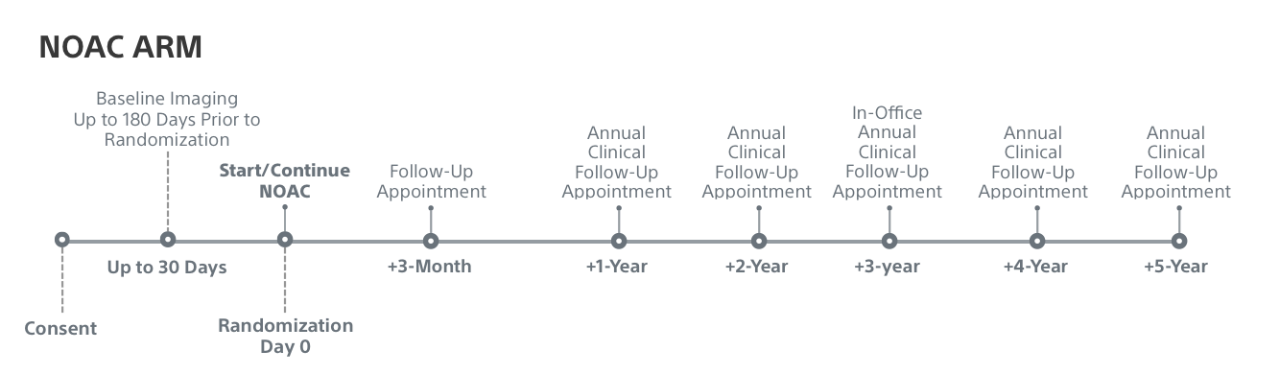
Trial Timeline
Patient Brochure
View the orderable patient-facing brochure created to give your trial candidates an overview of the CHAMPION-AF clinical trial.
Contact your WATCHMAN Representative to order patient brochures for your center.
Patient Selection

Find a Trial Site
Boston Scientific has selected US sites to be CHAMPION-AF investigational centers. If you have a candidate in mind, view the active trial list provided by clinicaltrials.gov, and reach out to a study physician near you to discuss next steps for referral.