ASAP-TOO Clinical Trial

The ASAP-TOO clinical trial will study the WATCHMAN™ Left Atrial Appendage Closure Implant. The trial is meant to study the implant in patients who are unable to take blood thinners.
The WATCHMAN Implant has been through several clinical trials already. It’s approved by the Food and Drug Administration (FDA) for people who can take blood thinners and has been implanted in more than 15,000 people worldwide.
In the ASAP-TOO clinical trial, physicians will test the ability of the WATCHMAN Implant to reduce stroke and systemic embolism risk in patients who cannot take blood thinners such as warfarin (Coumadin) for even a short period of time. This study has been approved by the FDA and the hospital’s review board.
What Will Happen in the Trial?
In the ASAP-TOO trial, some participants will receive the WATCHMAN Implant, and others will stay on their existing treatment. That’s called randomization, and it’s done so physicians can compare the effects of the implant versus not having the implant. It’s like a coin toss, so everyone has a fair chance of getting the implant. Both options include ongoing, high-quality support from cardiologists to help participants manage their atrial fibrillation.
If you’re randomized to receive the WATCHMAN Implant, you’ll have a one-time procedure to implant it. Whether you receive the implant or not, you’ll have regular follow-up appointments to make sure you’re doing well. If you agree to be in this study, you will be expected to visit your study doctor for all required examinations, study procedures, and follow-up tests.
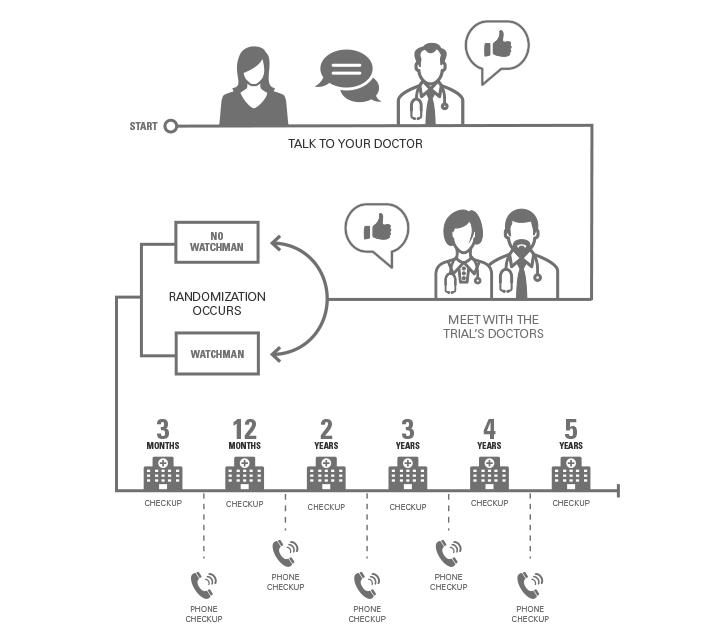
1. If you’re interested in the trial, begin by talking to your doctor.
2. If your doctor thinks you may be a good fit, you’ll meet with the trial’s doctors.
3. The trial's doctors will evaluate and confirm that you meet the study criteria.
4. The trial will use randomization to put you into one of two groups.
- Some people will have the WATCHMAN implanted.
- Others will continue their existing treatment.
5. Both groups will receive ongoing, high-quality support from the study cardiologists to help manage their atrial fibrillation.
- You’ll have visits at 3 months, 12 months, and years 2, 3, 4, and 5.
- You’ll have phone checkups every 6 months.
Does It Cost Anything to Participate in the ASAP-TOO Clinical Trial?

PATIENT SAFETY INFORMATION
The WATCHMAN Device is a permanent implant designed to close the left atrial appendage in the heart in an effort to reduce the risk of stroke. With all medical procedures there are risks associated with the implant procedure and the use of the device. The risks include but are not limited to accidental heart puncture, air embolism, allergic reaction, anemia, anesthesia risks, arrhythmias, AV (Arteriovenous) fistula, bleeding or throat pain from the TEE (Trans Esophageal Echo) probe, blood clot or air bubbles in the lungs or other organs, bruising at the catheter insertion site, clot formation on the WATCHMAN™ Closure Device, cranial bleed, excessive bleeding, gastrointestinal bleeding, groin puncture bleed, hypotension, infection/pneumonia, pneumothorax, pulmonary edema, pulmonary vein obstruction, renal failure, stroke, thrombosis and transient ischemic attack. In rare cases death can occur. Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of the WATCHMAN Device.