Boston Scientific's next innovation in Image Guided Programming
Vercise Neural Navigator 5 (VN5) Software makes it easier and more efficient to personalize your patients’ DBS journey.
Boston Scientific accounts are for healthcare professionals only.
Create an account to access online training and education on EDUCARE, manage your customer profile, and connect with customer support and service teams.
My Boston Scientific account
Access your online applications and manage your customer profile.
Quick Links
Call customer care
Vercise Neural Navigator 5 (VN5) Software makes it easier and more efficient to personalize your patients’ DBS journey.
Watch a preview of Vercise Neural Navigator 5.
To get answers to your questions and additional information, contact a DBS sales representative.
VN5 is Boston Scientific’s next innovation in Image Guided Programming. See stimulation to personalize therapy in each patient’s specific anatomy.*
20 minutes
average programming time1
56% reduction
in programming time**
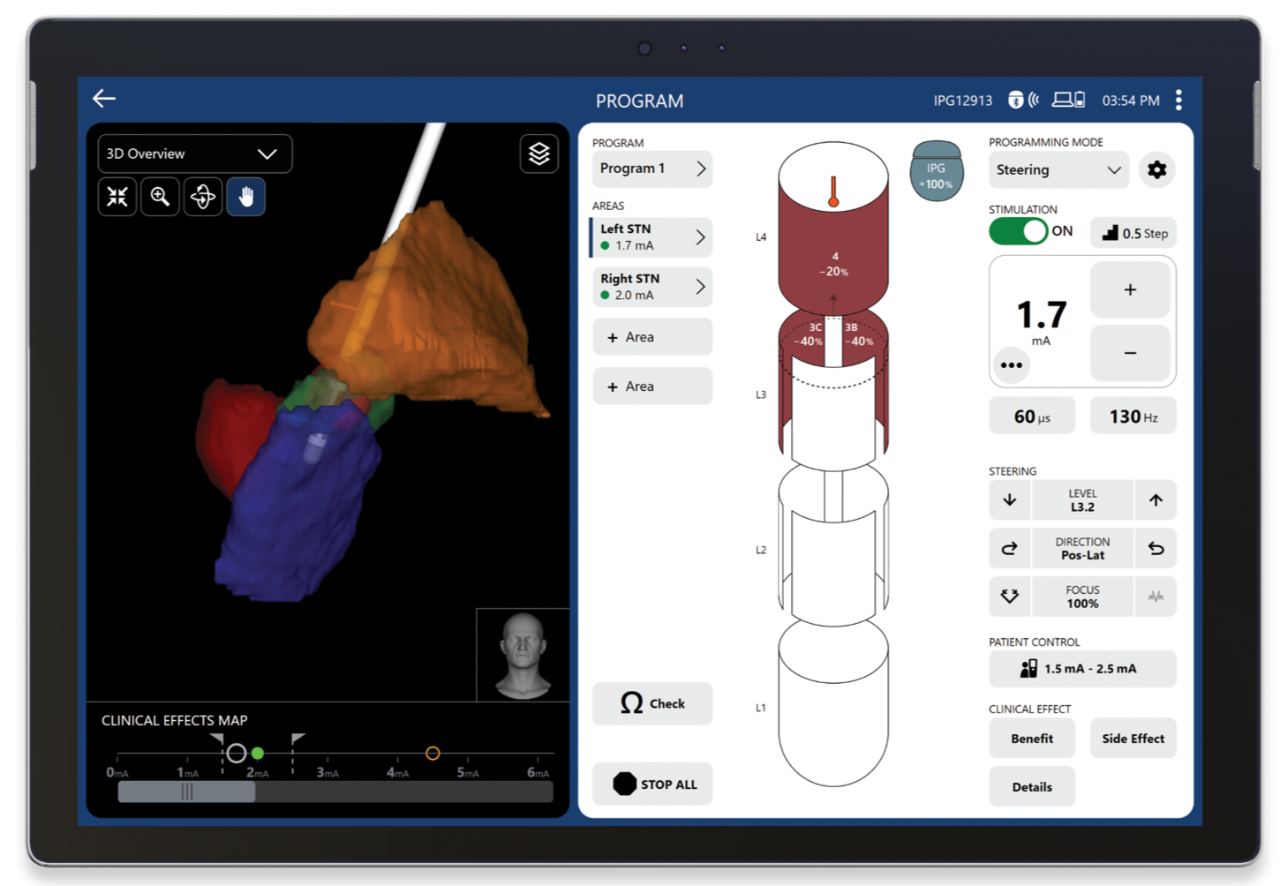
The new dashboard view shows your patient’s therapy at-a-glance for efficient decision-making.
Manage side effects as your patient’s disease progresses with Image Guided Programming.
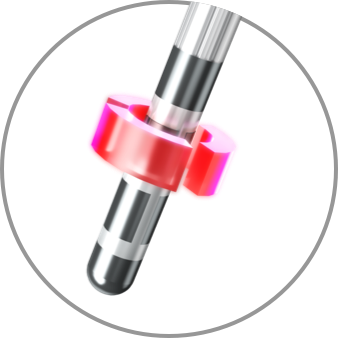
Monopolar

Bipolar
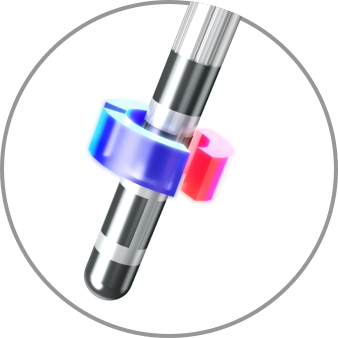
Bipolar Directional (Anodic Block)
Semi-bipolar and anodic stimulation capabilities are only available with Boston Scientific and are demonstrated to increase side effect threshold2,3 and improve efficacy.4
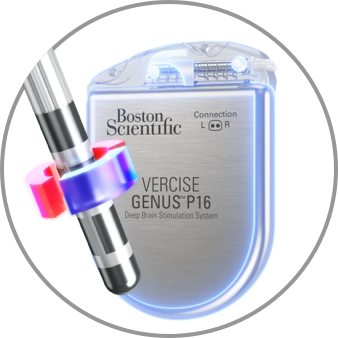
Semi-Bipolar
Anode divided between the IPG and the Lead
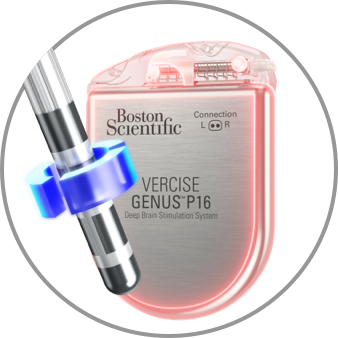
Anodic
100% Anode on the Lead, 100% Cathode on the IPG
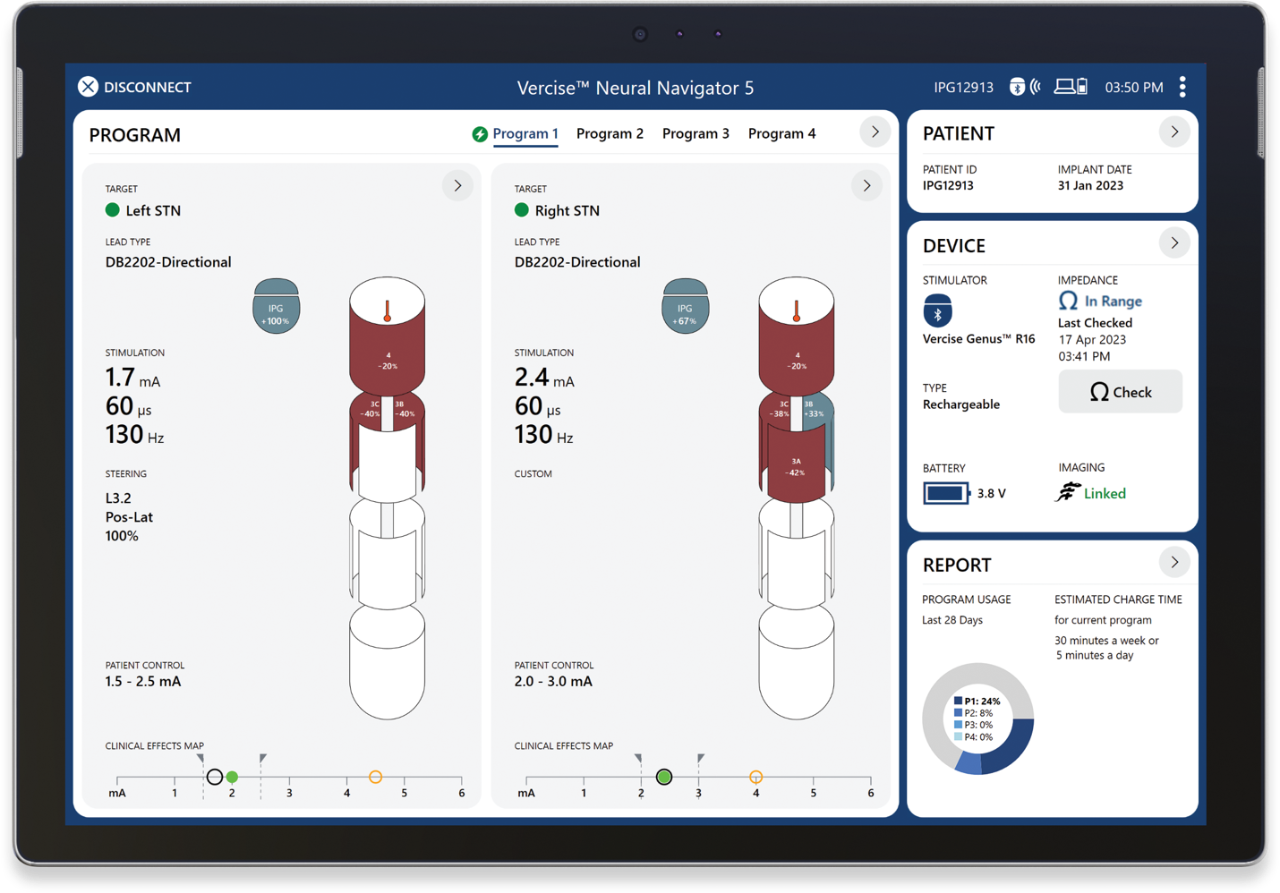
Stim-on Steering enables simple and accurate assessment of stimulation along the entire electrode.
Pre-plan personalized therapy with Guide XT to make your time with the patient even more efficient.
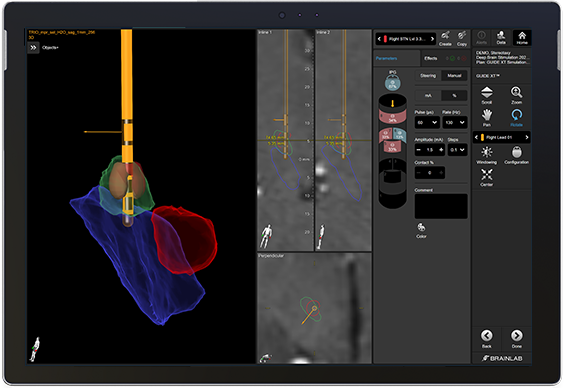
Pre-plan in Guide XT based on patient specific anatomy.
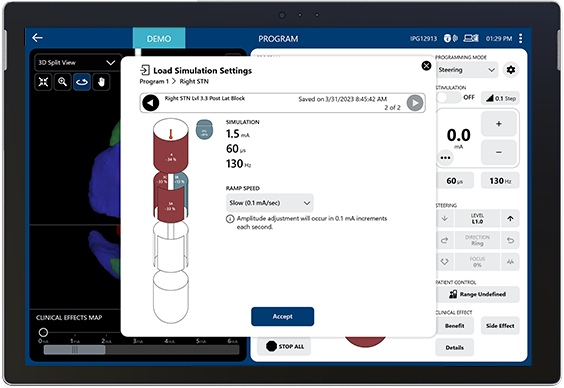
Load pre-planned stimulation when the patient is in the room.
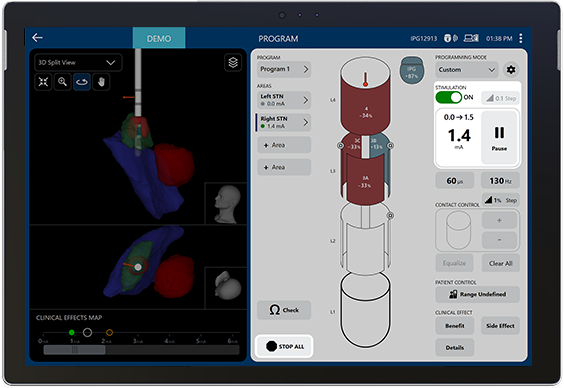
Automatically ramp amplitude as you clinically assess the patient.
If you are a patient looking for more information on DBS, visit DBSandMe.com.
Brainlab Elements Guide XT provides functionality to assist medical professionals in planning the programming of stimulation for patients receiving approved Boston Scientific Deep Brain Stimulation (DBS) devices.
*STIMVIEW™ XT Technology is a visual representation of the estimated stimulation field.
**Image Guided programming in PD patients enables a reduction in programming time compared with standard clinical based programming (p=39).
***Information for competitive devices excerpted from literature published by Medtronic (M982261A015 Rev A, M017563C002 Rev A, M939241A051 Rev A, M927170A073 Rev A, M017562C002 Rev A) and Abbott (ARTEN600150429 - B, ARTEN600102238 - A) Schüpbach, Michael & Chabardes, Stephan & Matthies, Cordula & Pollo, Claudio & Steigerwald, Frank & Timmermann, Lars & Vandewalle, Veerle & Volkmann, Jens & Schuurman, P.. (2017). Directional leads for deep brain stimulation: Opportunities and challenges. Movement Disorders. 32. 10.1002/mds.27096.
REFERENCE:
1. Lange F, et al. (2021). Reduced Programming Time and Strong Symptom Control Even in Chronic Course Through Imaging-Based DBS Programming. Front. Neurol. 12:785529.doi: 10.3389/fneur.2021.785529 n=10
2. Steffen, J. K., Reker, P., Mennicken, F. K., Dembek, T. A., Dafsari, H. S., Fink, G. R., Visser-Vandewalle, V., & Barbe, M. T. (2020). Bipolar Directional Deep Brain Stimulation in Essential and Parkinsonian Tremor. Neuromodulation: Technology at the Neural Interface, 23(4), 543–549. DOI: 10.1111/ner.13109
3. Reker, P., Dembek, T. A., Becker, J., Visser-Vandewalle, V., & Timmermann, L. (2016). Directional deep brain stimulation: A case of avoiding dysarthria with bipolar directional current steering. Parkinsonism & Related Disorders, 31, 156-158. https://doi.org/10.1016/j.parkreldis.2016.08.007
4. Kirsch, A. D., Hassin-Baer, S., Matthies, C., Volkmann, J., & Steigerwald, F. (2018). Anodic versus cathodic neurostimulation of the subthalamic nucleus: A randomized-controlled study of acute clinical effects. Parkinsonism & Related Disorders, 55, 61-67. https://doi.org/10.1016/j.parkreldis.2018.05.015
Results from different clinical investigations are not directly comparable. Information provided for educational purposes only.
Indication for Use: The Boston Scientific Vercise™ PC, Vercise Gevia™, Vercise Genus™ Deep Brain Stimulation Systems are indicated for use in:
-Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
-Bilateral stimulation of the internal globus pallidus (GPi) as an adjunctive therapy in reducing some of the symptoms of advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
-Unilateral thalamic stimulation of the ventral intermediate nucleus (VIM) is indicated for the suppression of tremor in the upper extremity. The system is intended for use in patients who are diagnosed with essential tremor or parkinsonian tremor not adequately controlled by medications and where the tremor constitutes a significant functional disability.
The Boston Scientific Vercise Deep Brain Stimulation System is indicated for use in:
-Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
Contraindications, warnings, precautions, side effects: The Boston Scientific Deep Brain Stimulation (DBS) Systems or any of its components, are contraindicated for: Diathermy as either a treatment for a medical condition or as part of a surgical procedure, Electroconvulsive Therapy (ECT) and Transcranial Magnetic Stimulation (TMS) as the safety of these therapies in patients implanted with the Boston Scientific DBS System has not been established, patients who are unable to operate the system, patients who are poor surgical candidates or who experience unsuccessful test stimulation. Patients implanted with Boston Scientific DBS System without ImageReady™ MRI Technology should not be exposed to Magnetic Resonance Imaging (MRI). Patients implanted with Vercise Gevia or Vercise Genus or Vercise Genus Mixed System with M8 Adapter or Vercise DBS Lead-Only System (before Stimulator is implanted) with ImageReady MRI Technology are Full Body MR Conditional only when exposed to the MRI environment under the specific conditions defined in ImageReady MRI Guidelines for Boston Scientific DBS Systems. Assess patients for the risks of depression and suicide. This assessment should consider both the risk of depression and suicide as well as the potential clinical benefits of DBS therapy. Monitor patients for new or worsening symptoms of depression, suicidal thoughts or behaviors, or changes in mood or impulse control and manage appropriately. Refer to the Instructions for Use provided with the Boston Scientific DBS Systems or BostonScientific.com for potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.