*MR-Conditional when all conditions of use are met.
**Information for competitive devices excerpted from the literature published by Medtronic (M982261A015 Rev A, M939241A051 Rev A, M013074C001 Rev B, M982097A013 Rev A, M13075C001 Rev B, M019192C002 Rev A) and Abbott (ARTEN600150429 - B, ARTEN600102238 - A, ARTEN600266398 -A, ARTEN600308953 -A, ARTEN600308947 -A), and Schüpbach, Michael & Chabardes, Stephan & Matthies, Cordula & Pollo, Claudio & Steigerwald, Frank & Timmermann, Lars & Vandewalle, Veerle & Volkmann, Jens & Schuurman, P.. (2017). Directional leads for deep brain stimulation: Opportunities and challenges. Movement Disorders. 32. 10.1002/mds.27096. Steffen, J. K., Reker, P., Mennicken, F. K., Dembek, T. A., Dafsari, H. S., Fink, G. R., Visser-Vandewalle, V., & Barbe, M. T. (2020). Bipolar Directional Deep Brain Stimulation in Essential and Parkinsonian Tremor. Neuromodulation: Technology at the Neural Interface, 23(4), 543–549. DOI: 10.1111/ner.13109 Reker, P., Dembek, T. A., Becker, J., Visser-Vandewalle, V., & Timmermann, L. (2016). Directional deep brain stimulation: A case of avoiding dysarthria with bipolar directional current steering. Parkinsonism & Related Disorders, 31, 156-158. https://doi.org/10.1016/j.parkreldis.2016.08.007 Kirsch, A. D., Hassin-Baer, S., Matthies, C., Volkmann, J., & Steigerwald, F. (2018). Anodic versus cathodic neurostimulation of the subthalamic nucleus: A randomized-controlled study of acute clinical effects. Parkinsonism & Related Disorders, 55, 61-67. https://doi.org/10.1016/j.parkreldis.2018.05.015. Boston Scientific (Vercise ™ Neural Navigator 5 Software Programming Manual MP92736308-01)
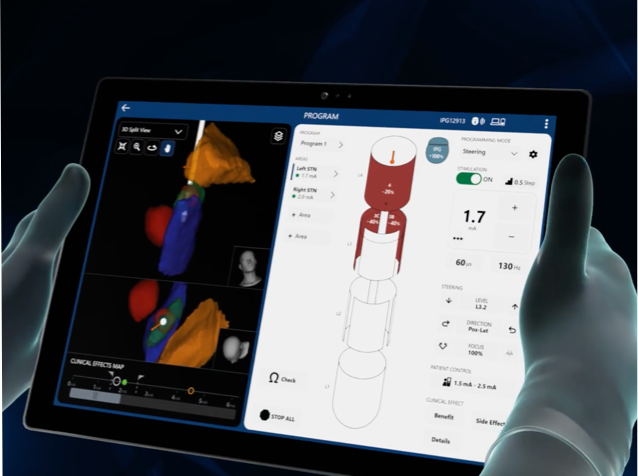
†STIMVIEW™ XT Technology is a visual representation of the estimated stimulation field.
REFERENCES:
- Image Guided programming in PD patients enables a reduction in programming time compared with standard clinical based programming (p=39). Lange F, Et al. Reduced Programming Time and Strong Symptom Control Even in Chronic Course Through Imaging-Based DBS Programming. Front Neurol. 2021 Nov 8;12:785529. N=10
- Yu et al. (2013). “Characterizing Rechargeable IPG Charge Cycle Time in DBS.” NANS 2013 Poster.
- 2023. Real-World Outcomes Using DBS Systems with Directionality and Multiple Independent Current Control - US Experience. North American Neuromodulation Society, January 2023, Las Vegas, NV. (N = 49)
A System that includes the Vercise™ PC, Vercise Gevia™, or Vercise Genus™ IPG and Vercise Cartesia™ Directional Lead(s) forms the Vercise Directional System