POLARx™
Cryoablation System
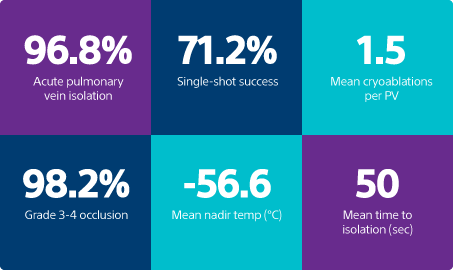
*Only ablations with duration >60S included in ablation counts

Freedom from atrial arrhythmias
at follow-up (12 months) after using POLARx FIT

Major adverse events
including esophageal fistulas, persistent phrenic nerve palsy and PV stenosis

High-grade occlusion rate
resulting in either Grade 3 or Grade 4 occlusion level


POLAR ICE multicenter study

ANTARCTICA observational study

Acute PV isolation

Real-time PVI visualization

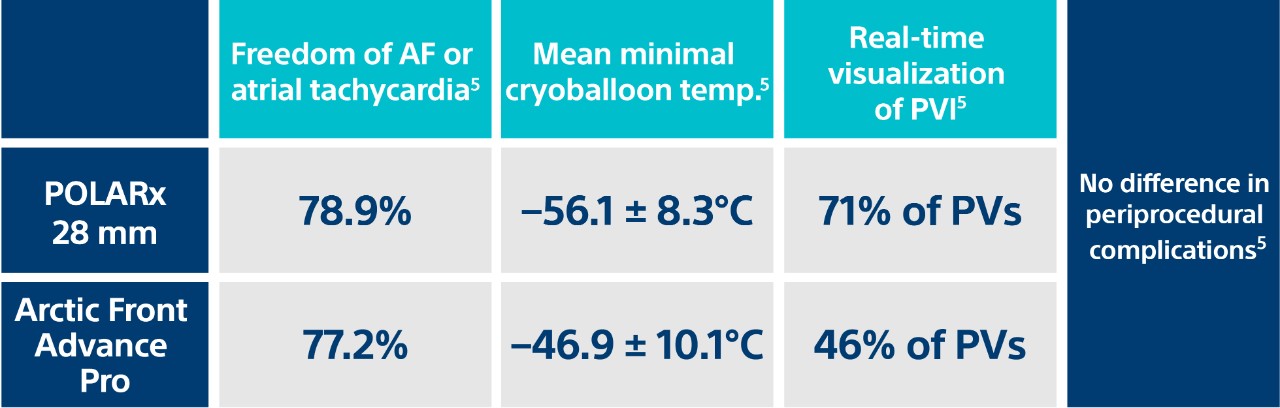
Freedom from recurrence
at 226 ± 115 days

Performance vs. existing cyroablation systems
Prospective ICE AGE 1 Study

U.K. Comparative Study

Reported phrenic nerve palsy6

Complication rate6
Procedure time6

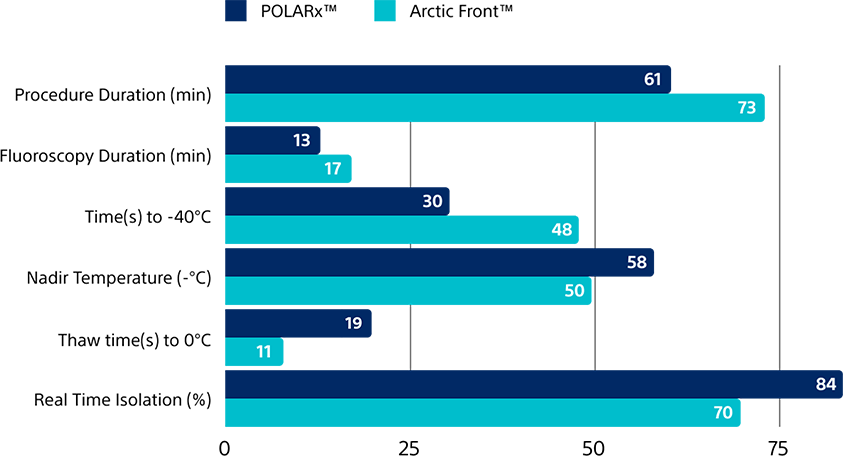
Propensity Score-Matched Comparison

Faster procedure time
vs. Arctic Front Advance
(61 min. vs. 73 min.)

Faster to -40˚C
vs. Arctic Front Advance
(30 sec. vs. 48 sec.)

Greater real-time isolation rate
vs. Arctic Front Advance
(84% vs. 70%)
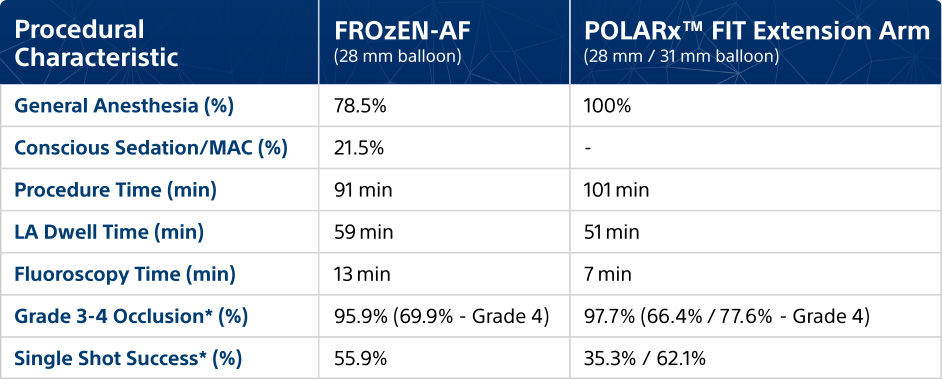
Procedural characteristics

Studies comparing POLARx™ and other cryoablation systems

Advance your understanding
Continue exploring POLARx technology on EDUCARE, Boston Scientific’s online medical education platform.
References:
1. Ellenbogen KA, Mittal S, Varma N, et al. One-year outcomes of pulmonary vein isolation with a novel cryoballoon: Primary results of the FROZEN AF trial. J Cardiovasc Electrophysiol. 2024 March 6. doi.org/10.1111/jce.16220
2. Luik A, Anic A, Asmundis C, et al. Long-term success rates of a stable, low pressure cryoballoon for the treatment of paroxysmal atrial fibrillation: Results of the prospective, international, multicenter POLAR-ICE Study. Presented at: 2023 ESC Congress, Aug. 25-28, 2023; Amsterdam, Netherlands.
3. Martin CA, Tilz RRR, Anic A, et al. Acute procedural efficacy and safety of a novel cryoballoon for the treatment of paroxysmal atrial fibrillation: Results from the POLAR ICE study. J Cardiovasc Electrophysiol. 2023 Apr;34(4):833-840. doi: 10.1111/jce.15861. Epub 2023 Feb 23. PMID: 36786515.
4. Heeger CH, Pott A, Sohns C, et al. Novel cryoballoon ablation system for pulmonary vein isolation: multicenter assessment of efficacy and safety-ANTARCTICA study. Europace. 2022 Dec 9;24(12):1917-25
5. Heeger C-H, Popescu SS, Inderhees T. et al. Novel or established cryoballoon ablation system for pulmonary vein isolation: the prospective ICE-AGE-1 study. Europace. 2023 Aug 2;25(9):euad248. doi: 10.1093/europace/euad248.
6. Honarbakhsh S, Martin CA, Mesquita J, et al. AF cryoablation is an effective day case treatment: The United Kingdom PolarX versus AFA experience. EP Europace. Sept 21, 2023. euad286. doi.org/10.1093/europace/euad286
7. Mojica J, Lipartiti F, Al Housari M, et al. Procedural safety and efficacy for pulmonary vein isolation with the novel POLARx™ Cryoablation System: A propensity score matched comparison with the Arctic Front™ Cryoballoon in the setting of paroxysmal atrial fibrillation. J Atr Fibrillation. 2021 Jun 30;14(1):20200455.
† Updated analysis with corrected data.