
Discover how your transvenous implantable cardioverter defibrillator (TV-ICD) or subcutaneous implantable cardioverter defibrillator (S-ICD) system works to monitor and treat dangerously fast ventricular heart rhythms so you can live a more active life.
What is an ICD?
An implantable cardioverter defibrillator, commonly known as an ICD, is a device designed to administer lifesaving therapy in the event of a sudden cardiac arrest (SCA). When the ICD senses a dangerously high heart rate, it will send an electrical pulse to your heart to reset its normal rhythm and allow your heart to resume pumping blood through your body. This is known as defibrillation. ICDs have been used for decades and have prolonged hundreds of thousands of lives.
Types of ICDs
There are two types of ICDs being implanted today:
- Transvenous ICD systems, also known as TV-ICDs
- Subcutaneous ICDs, such as the EMBLEM™ S-ICD
Both types of ICDs sense when the heart rate is dangerously fast and can deliver a shock to the heart to restore a normal heartbeat.
TV-ICD implant procedure
- A TV-ICD device is typically implanted in the left shoulder area, near the collarbone.
- Using X-ray imaging, the leads are fed through a vein into the heart and across the heart valve.
- Depending on your heart condition, one or two leads will be placed in the heart. Once the leads are put in place, they are attached to the heart wall for optimal connectivity.
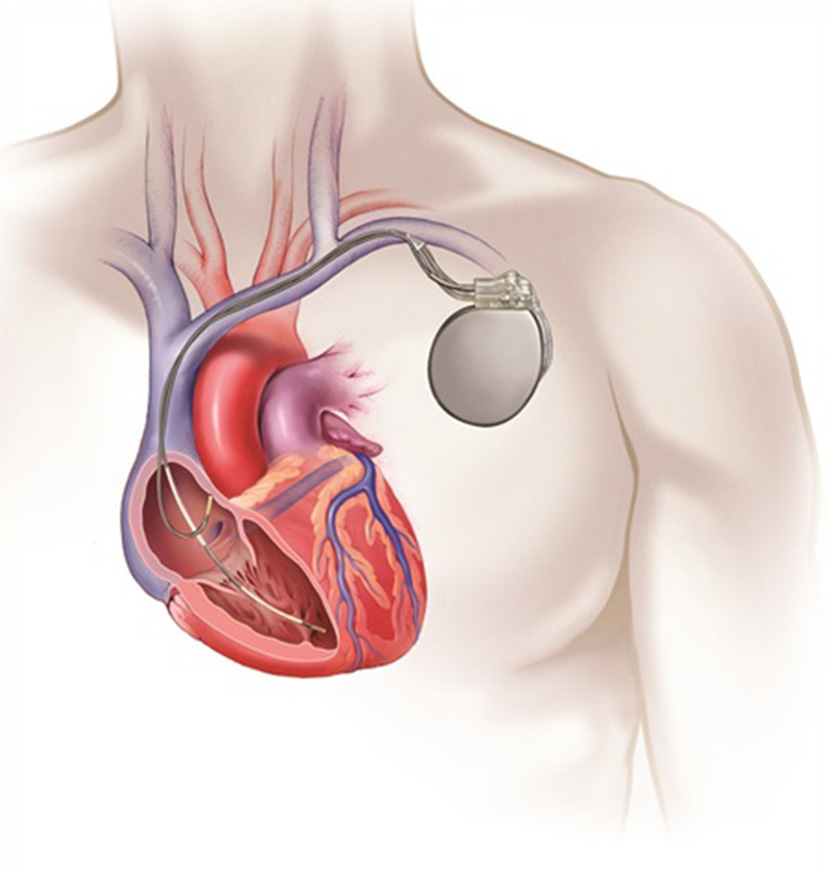
An implanted TV-ICD system.
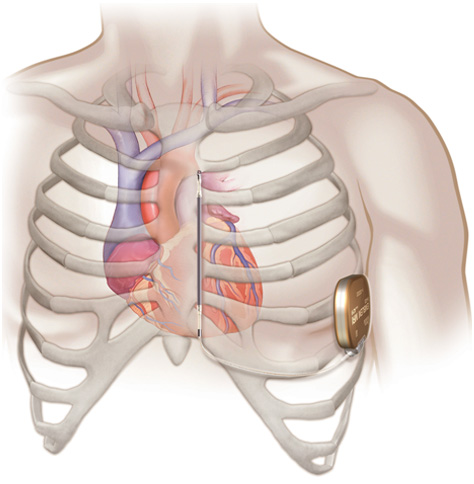
An implanted subcutaneous S-ICD system.
EMBLEM™ S-ICD procedure
- Unlike a TV-ICD device, the pulse generator in an S-ICD is typically implanted on the left side of the chest next to the rib cage and the lead is implanted just under the skin above the breastbone.
- The EMBLEM S-ICD electrode is placed under the skin and the system delivers therapy without the need for wires implanted in the heart.
- EMBLEM S-ICD leaves the heart and blood vessels untouched and intact.
About shock therapy
While you won't feel any noticeable sensations when your TV-ICD or S-ICD is monitoring your heart, shock therapy for an arrhythmia may be very noticeable. It's important to know what to expect in advance.
Preparing for shock therapy
Before you experience symptoms or receive a shock, talk to your health care team about a plan for contacting them and, if necessary, emergency personnel. It's also a good idea to consider the following suggestions:
- If possible, have someone who is prepared to perform cardiopulmonary resuscitation (CPR) should you need it and stay with you through the event
- Make sure a friend or family member knows to call 911 if you remain unconscious
- If you are conscious but don't feel well after a shock, have someone call your health care provider
- If you feel fine after a shock and no more symptoms appear, it may not be necessary to seek medical help immediately. However, follow your health care provider's instructions for when to call his or her office
- It's possible that you could feel symptoms of an arrhythmia but not receive therapy. This depends on the programmed settings of your device. For example, an arrhythmia may cause symptoms, but it may not be fast enough for your device to deliver therapy. In any case, if your symptoms are severe or continue for more than a minute or so, you should seek immediate medical attention
How shock therapy feels
With both TV-ICDs and S-ICDs, people have reported a wide range of experiences as a result of receiving a shock, from a mild thump to a kick in the chest. While the shock may be painful, it is over in an instant. This means your ICD or S-ICD device is monitoring and responding to dangerous heart rhythm irregularities. The sensation you feel may vary based on the therapy your device delivers.
Defibrillation
If your arrhythmia is very irregular and fast, your device can deliver a high-energy shock to stop the arrhythmia and return your heart to its normal rhythm. Many patients faint or become unconscious shortly after a very fast VT or VF rhythm begins. As a result, many patients do not feel these high-energy shocks. Some say the sudden but brief sensation feels like a kick in the chest. This sensation will only last for a moment. While many find the shock reassuring, other patients may be upset for a short time after the shock is delivered.
Your TV-ICD or S-ICD battery
Like any battery, the energy in your TV-ICD or S-ICD device will naturally decrease over time. The battery’s life is affected by the specific device you have, the setting your doctor programs, and how much therapy you receive.
For more information on TV-ICD battery, click here.
For more information on S-ICD battery, click here.
TV-ICD and S-ICD implant risks
Every surgical procedure has some risks. This includes infection and bleeding. In the rare case your ICD becomes infected, removal may be required. Because the TV-ICD leads are inside the heart, removal is more complicated than removing the S-ICD leads, which are under the skin. While uncommon, your ICD could deliver a shock when not necessary, which is called an “inappropriate shock.” Ask your doctor for more information about the risks associated with ICD devices. For more detailed information you can also refer to the following pages in your respective ICD Patient Handbook.
For S-ICD go to pages 31-33 here.
For TV-ICD go to pages 44-48 here.