What to expect from recovery
A full recovery from an implantable defibrillator (TV-ICD and S-ICD) procedure can take a few days or a few months. Find general recovery tips and guidelines below, and be sure to follow your doctor's post-operative instructions and talk to him or her about resuming normal activities based on your specific situation.

Post-recovery guidelines
After your procedure, your health care team will give you post-operative directions, which may include:
- Avoid activities that involve heavy lifting or rough contact that could result in blows to your implant site and to allow your incision time to heal
- Call your doctor if you have any swelling, redness or discharge around your incision, or if you notice anything unusual or unexpected, such as developing a fever that does not go away in two or three days
- Call your doctor if you hear any beeping sounds from your device as this indicates your device needs to be checked immediately. Carry your Medical Device ID Card with you at all times
As you recover from a TV-ICD or S-ICD implant procedure, it’s important to follow your doctor’s instructions, including:
- Questions you may have about your device, heart rhythm, or medication
- Keep tight clothing and jewelry away from the skin over your device
- Avoid rubbing your device or the surrounding chest area
- Follow your doctor’s instructions for exercise and bathing
- Tell your other doctors, dentists, and emergency personnel that you have an implanted device and show them your Medical Device Identification Card
Activities and exercises
Your doctor will help you decide what level of activity is best for you and which activities you should avoid while recovering. Consider avoiding:
- Strenuous activity, especially lifting and other activities that use your upper body. This will give the incision where the device was implanted time to heal
- Rough contact that could result in blows to your implant site
- Lifting heavy objects until instructed by your doctor
- Arm movements that could affect your lead system as directed by your doctor
Driving after your ICD procedure
The driving laws where you live, and the symptoms caused by your arrhythmia will often determine whether you can drive. Be sure to ask your health care provider about any driving restrictions you may have.
Whether you're a driver or passenger, you may want to find padding to cover your surgery site and make wearing a seatbelt more comfortable. You may find pads in local retail stores that will provide comfort for your pocket site.
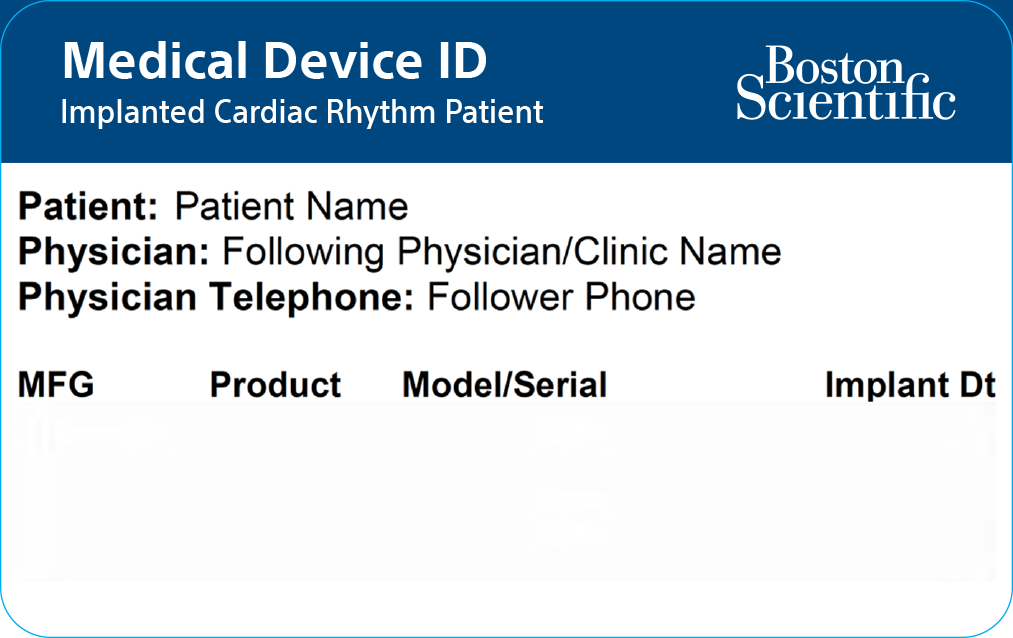
Medical device ID card
Whether you’re going away for the weekend or running a quick errand, it’s important to carry your Medical Device Identification Card with you at all times. Your Medical Device ID Card contains your name, your doctor’s name and phone number, and the model numbers of your device and leads. In an emergency, the card will alert medical and security personnel that you have an implanted device. You will be given a temporary Medical Device ID Card when you receive your TV-ICD or S-ICD device. Your permanent card will be mailed to your home within two weeks after your implant.
Medical Device ID Card - Front
Medical Device ID Card - Back

Regular follow-up visits
It’s important to maintain all follow-up visits, even if you’re feeling well. These appointments will help your doctor check your TV-ICD or S-ICD device and overall health on a regular basis.
A typical follow-up appointment takes about 20 minutes. During your visit, your doctor or nurse will use a programmer to check your device. They will examine your TV-ICD or S-ICD device’s memory, evaluate how much energy is left in your battery and check to see if you had any arrhythmia episodes since your last visit. If necessary, they will adjust your device’s programmed settings.
Moving or selecting a new doctor
Please tell us if you move or get a new doctor. You can use our online patient portal or call us at 1-800-728-3282 to update your record, and we will send you a new ID card.
When to call a doctor
Your doctor will provide guidelines for when you should contact him or her. In general, call your doctor if you:
- Notice anything unusual or unexpected, such as new symptoms or symptoms like the ones you experienced before you received your device
- Have any redness, swelling, or drainage from your incisions
- Start a fever that does not go away in two or three days
- Receive any arrhythmia therapy from your device and have been instructed to call
- Develop symptoms of an abnormal heart rhythm and have been instructed to call
- Hear any beeping sounds from your device. This indicates that your device needs to be checked immediately
- Have questions about your device, heart rhythm, or medications
- Plan to travel or move so you can work with your doctor to develop a follow-up plan while you are away