The SENTINEL™ Cerebral Protection System
The SENTINEL Cerebral Protection System (CPS) is the first and only device in the U.S. to help protect patient from the risk of stroke during a Transcatheter Aortic Valve Replacement (TAVR) procedure.
Boston Scientific accounts are for healthcare professionals only.
Create an account to access online training and education on EDUCARE, manage your customer profile, and connect with customer support and service teams.
My Boston Scientific account
Access your online applications and manage your customer profile.
Quick Links
Call customer care
The SENTINEL Cerebral Protection System (CPS) is the first and only device in the U.S. to help protect patient from the risk of stroke during a Transcatheter Aortic Valve Replacement (TAVR) procedure.
Watch as Professor Bernard Prendergast and Professor Simon Redwood protect their patient from a potentially harmful piece of cerebral embolic debris that they captured during a TAVR case at St. Thomas’ Hospital in London.
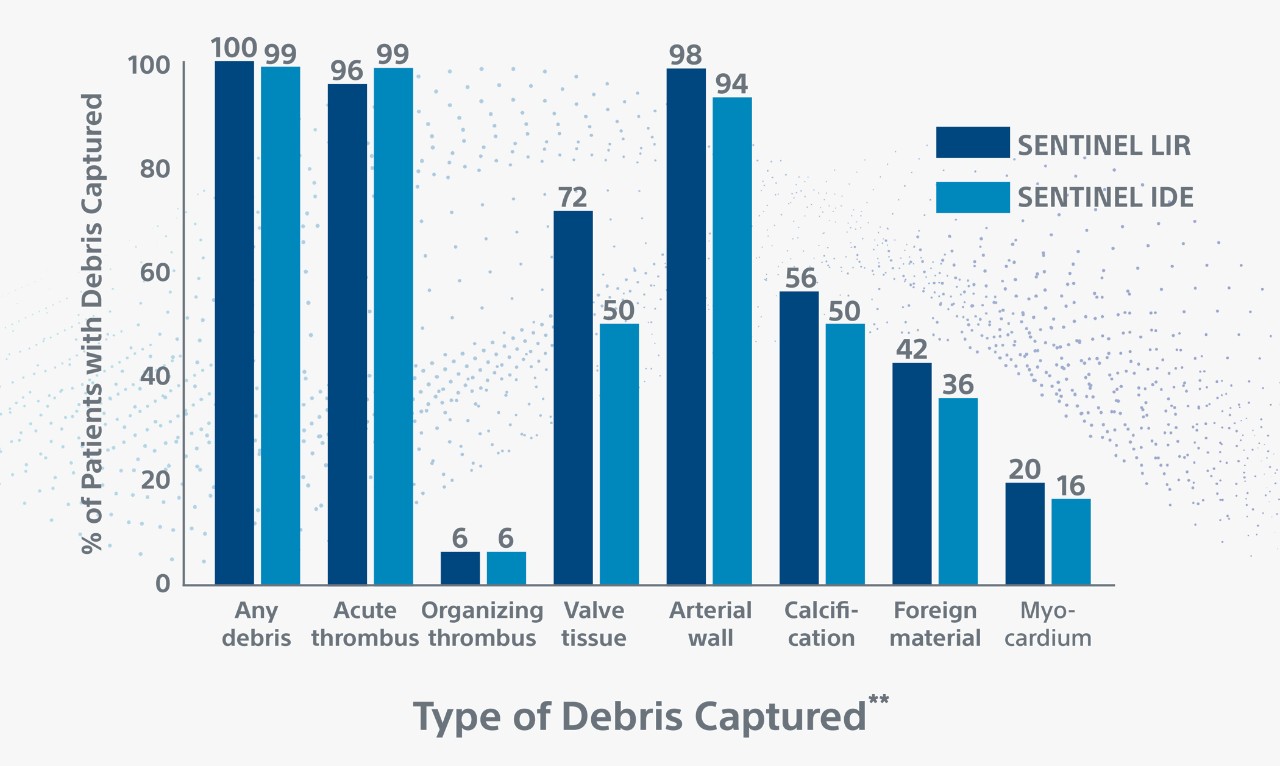
In 99% of procedures, SENTINEL was shown to capture and remove stroke-causing embolic debris such as arterial wall pieces, valve tissue, calcified and foreign material.1,2

The SENTINEL CPS features a strong safety profile. SENTINEL demonstrated excellent safety with high rates of device delivery/retrieval (94.4%) and very low rates of vascular complications (0.1%).3
The SENTINEL CPS captures and removes debris loosened during TAVR procedures. It is delivered percutaneously immediately before a TAVR procedure and filters 90% of the blood flow to the brain.
Younger patients have a higher need for cerebral protection as they begin their TAVR journey and have potential to experience greater quality of life impact. Stroke is a key risk to any TAVR patient, no matter their age or clinical background.
Characterization of cerebral emboli capture using SENTINEL during TAVR.4
STUDY DESIGN: N = 50 low-intermediate surgical-risk patients; Real-world, multicenter, prospective registry.
Clinical highlights
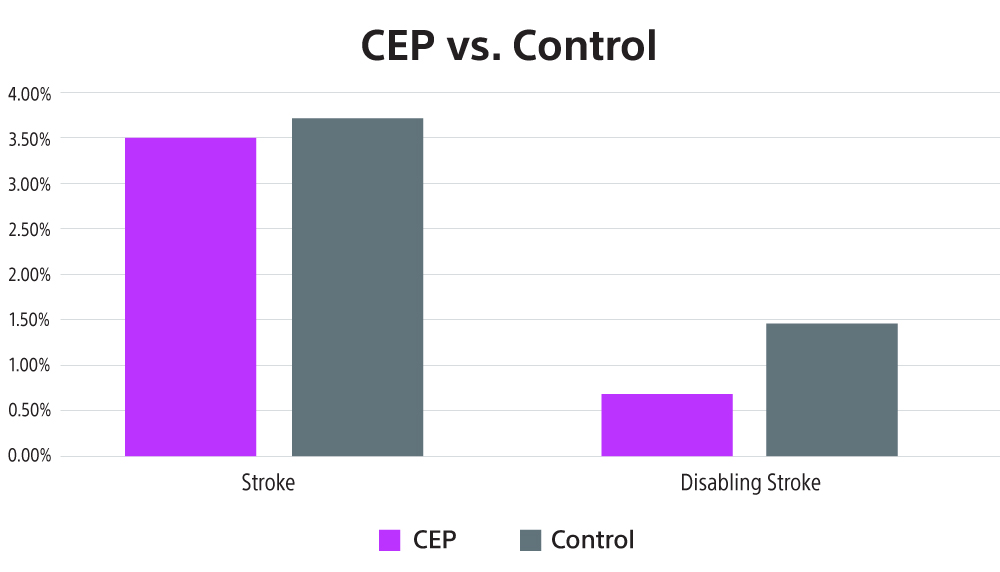
In a meta-analysis data from seven randomized control trials were analyzed with 4,031 patients included. It was shown that patients in the CEP group had lower risk of stroke (OR 0.81, 95% CI [0.57-1.14]; p=0.23) and lower risk of disabling stroke (0.45, [0.23-0.87]; p=0.02) than those in the control group.6
*Participating sites: Cleveland Clinic (Cleveland, OH), UPMC Pinnacle (Harrisburg, PA), Columbia (New York, NY), Atrium Health (Charlotte, NC)
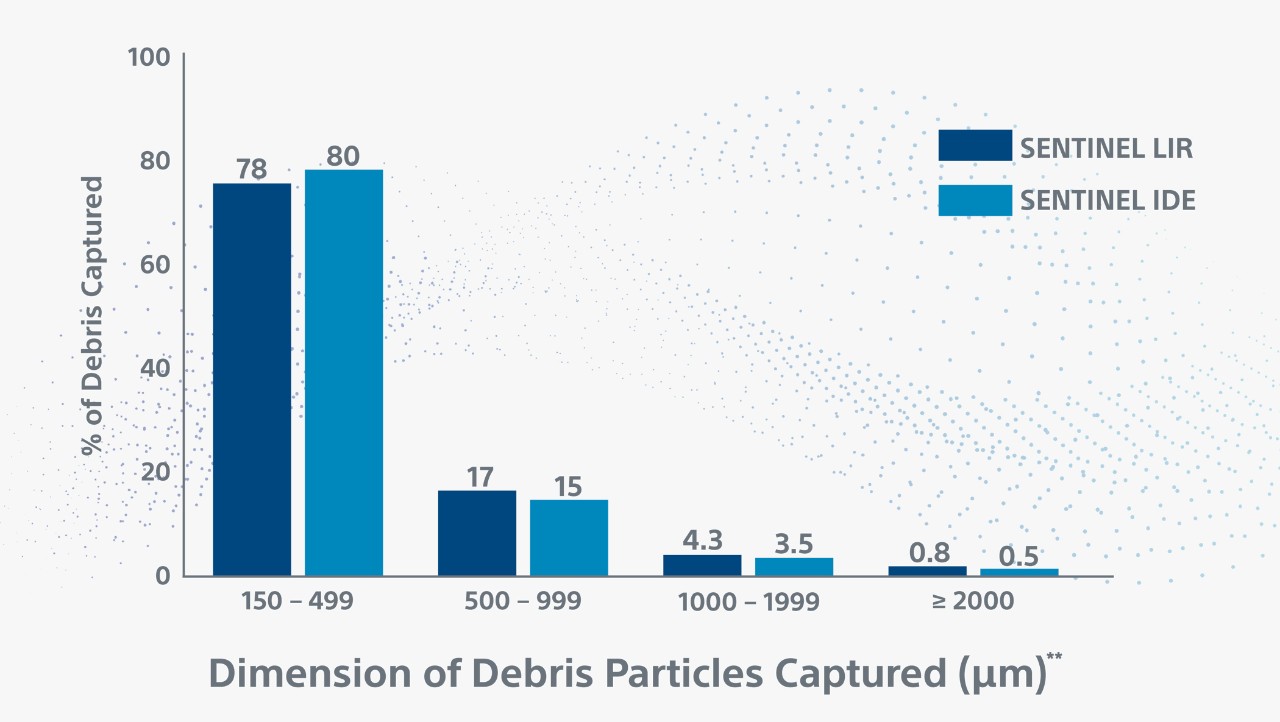
** Debris included arterial wall, acute thrombus, valve tissue, calcification and foreign material. Myocardium, organizing thrombus and necrotic core were observed less frequently. Majority (78%) of debris particles were 150-499 in maximum diameter; Larger size particles (≥1000), which can cause significant vessel obstruction, were present in 5% of patients.
1. SENTINEL IDE Trial. Data presented at SENTINEL FDA Advisory Panel, Feb 23, 2017.; 2. Van Mieghem N., TVT 2018 (includes TIA); 3. Stripe, B. PCR LV 2019; 4. Rinaldi, TCT 2018. 5. Chakravarty T., TCT 2018; 6. Seeger J., et al., JACC Cardiovasc Interv. 2017.
2. All photographs taken by Boston Scientific. Illustrations for informational purposes only – not indicative of actual size or clinical outcome.
3. Kapadia, S. PROTECTED TAVR Trial data presented at TCT 2022.
4. The SENTINEL Low-Intermediate - Registry (LIR): Characterization of Cerebral Emboli Capture Using the Sentinel Device during TAVR in Low to Intermediate Risk Patients. Presented at CRT 2021 by Dr. Aloke Finn, CV Path Institute.
5. SENTINEL IDE Trial. Data presented at SENTINEL FDA Advisory Panel, February 23, 2017.
6. Abstract by Palicherla et al presented at ACC 2023.
All photographs taken by Boston Scientific. Illustrations for informational purposes only – not indicative of actual size or clinical outcome.
All trademarks are property of their respective owners.