Every patient deserves protection from disabling stroke
Routine Cerebral Embolic Protection in Transcatheter Aortic Valve Implantation: The British Heart Foundation PROTECT-TAVI Trial1
The British Heart Foundation PROTECT-TAVI (BHF P-TAVI) trial is a United Kingdom-based, prospective, open-label, blinded, outcome-adjudicated, multicenter, randomized, controlled trial that evaluated the routine use of a cerebral embolic protection device – the SENTINELTM Cerebral Protection System device – to prevent stroke in patients with severe aortic valve stenosis (AS) undergoing TAVI.

This landmark trial is the largest randomized transcatheter aortic valve implantation (TAVI) trial to date, with 7,635 patients enrolled at more than 30 sites in the United Kingdom who were randomized 1:1 – patients protected with the SENTINEL Cerebral Protection System (CPS) versus no use of the SENTINEL device during TAVI.
- A total of 20 patients from the CEP group and 21 from the control group were withdrawn from the study resulting in the modified intention-to-treat (ITT) population (population used to assess the primary endpoint): CEP group n=3,795 and control group n=3,799.
- Standardized training on use of the SENTINEL device was required for sites to be eligible to enroll in the trial.
- The trial enrolled patients with AS who were scheduled to undergo TAVI, and in the opinion of the treating physician, were clinically and anatomically suitable for treatment with the SENTINEL device.
- There was no mandated screening of the aortic arch anatomy and patient selection was left to the discretion of the treating physician.
- There were no specific exclusion criteria
The primary outcome was defined as stroke at 72 hours post-TAVI (or at hospital discharge, if sooner)
- Stroke was defined as a new or worsened focal or global neurological deficit of presumed vascular origin, either ischemic or hemorrhagic, occurring after randomization and persisting for more than 24 hours or leading to death.
- For patients with a stroke outcome, stroke severity was assessed according to the National Institutes of Health Stroke Scale (NIHSS) at the time of initial assessment; Severe stroke was defined as a NIHSS of 10.
- The level of post-stroke disability was assessed using the modified Rankin Scale (mRS) at 6-8 weeks post-TAVI.
- Disabling stroke was defined as an mRS of ≥2 and an increase from the pre-procedure baseline mRS of at least 1 point.
- Both filters of the CEP device were fully and correctly deployed for the duration of the procedure in 3,058 of 3,768 (81.2%) allocated to CEP group. CEP was not deployed according to the protocol in 710 of 3768 (18.8%) of cases (Table 1).

- The primary and secondary outcomes were assessed in the modified ITT population which included all randomized patients whose TAVI procedure was started, according to the group to which they were assigned, irrespective of whether they received the intervention as allocated or not.
- Baseline demographics, clinical characteristics, and procedural details were balanced between the TAVI with CEP and control groups:
- Mean age 81.2 years, female sex 38.7%, median EuroSCORE II 2.4%.
- The incidence of the primary outcome of stroke at 72 hours post-TAVI or at discharge (if sooner) was comparable among both groups (refer to Figure 1).
 Figure 1
Figure 1
- The incidence of secondary outcomes of disabling stroke (within 6-8 weeks), as well as death, non-fatal stroke, and transient ischemic attack (TIA) within 72 hours or discharge (if sooner) were also comparable among both groups (refer to Figure 2).
 Figure 2
Figure 2
 Figure 2
Figure 2
- Clinical complications and adverse events were similar between the CEP and control groups (refer to Table 2 below).
- Serious adverse events occurred in 24 of 3,798 patients (0.6%) in the CEP group and 13 of 3,803 patients (0.3%) in the control group.
- The higher number of adverse events in the CEP group was mainly driven by COVID-19, fall or accidental injury, gastrointestinal or abdominal, infection, procedural complication (4 cases), and respiratory. Only three cases were of cardiovascular-related causes.

To address non-compliance with the allocated treatment (being use CEP or no CEP), researchers used a method called Complier Average Causal Effect (CACE) where they predicted how likely patients were to receive treatment based on their original assignment (receive CEP or not receive CEP), and used this prediction to measure the treatments’ actual effect on the primary outcome (will use of CEP reduce or not reduce stroke). This method helps to provide a more accurate estimate of the treatments’ real impact.
- The CACE analysis did not demonstrate any difference in outcome between the two treatments groups.
- - Occurrence of all-stroke (difference -0.1%, 95% confidence interval – 0.9% to 0.7%).
- - Occurrence of disabling stroke (difference -0.2%; 95% confidence interval -0.8% to 0.5%).
- The CACE analysis did not demonstrate any difference in outcome between the two treatments groups.
A pre-specified subgroup analysis was performed to identify an interaction between the randomized treatment and any specific patient subgroup (including an adjudication for age and sex). Results showed that the incidence of stroke at 72 hours, or hospital discharge if sooner, was similar between both groups.

Study Conclusions
In the study population, routine use of the SENTINEL device in patients undergoing TAVI was not effective in reducing stroke.
Additional clinical data is needed to identify which patient sub-groups are at a higher risk of stroke.
TAVR Post-hoc Analysis: Outcomes by Geographic Region
Cerebral Embolic Protection by Geographic Region2,3
PROTECTED TAVR Trial Background: Overall Cohort
PROTECTED TAVR (PTAVR) is a randomized control trial (RCT) including N=3,000 patients with aortic stenosis enrolled at 51 sites globally and randomized 1:1 to TAVR with SENTINEL (n=1,501) or TAVR without SENTINEL (control group, n=1,499) (Feb 2020-Jan 2022).
Primary Endpoint:
All-stroke through 72 hours post-TAVR procedure or hospital discharge (whichever comes first).
Main PTAVR Overall Cohort Findings:
Primary endpoint was not met but there was a numerical trend towards lower rate of all-stroke in patients treated with SENTINEL, with a 21% relative risk reduction in all-stroke at discharge or 72 hrs (p=0.3).
Secondary analyses of disabling stroke showed a 60% relative risk reduction in disabling stroke at discharge or 72 hrs (p=0.02) in patients treated with SENTINEL.
Post-hoc US Cohort Study:
- Objective: To explore regional differences in the association of CEP utilization with stroke outcomes in patients undergoing TAVR.
The post-hoc analysis evaluated outcomes in patients enrolled in the US (N=1,833; TAVR with SENTINEL n=914 or TAVR without SENTINEL n=919) and OUS (Outside of the US: Europe and Australia) (N=1167; TAVR with SENTINEL n=587 or TAVR without SENTINEL n=580).
- Primary endpoint and secondary analyses were similar to the PTAVR overall cohort.
Patient Characteristics:
Patients in US cohort were younger, more predominantly male, had a lower prevalence of atrial fibrillation, and a higher prevalence of bicuspid aortic valves, diabetes, and peripheral vascular disease compared to OUS cohort.
As for the procedural practices, there was a higher usage of balloon expandable valves for TAVR in the US (77.7% US vs 42.4% OUS; p<0.001) and a lower rate of pre-dilation (27.5% US vs 60.1% OUS; p<0.001) versus OUS, reflective of the current clinical practice trends in both regions.
Stroke Outcomes - Baseline Characteristics:
Primary Endpoint:
In the US cohort, the primary endpoint showed a significant reduction in all-stroke for TAVR with SENTINEL (1.3.%) vs TAVR without SENTINEL (2.6%), representing a 50% relative risk reduction in all-stroke at discharge or 72-hours (p=0.045).
No significant difference was observed in the OUS cohort (TAVR with SENTINEL 3.7% vs TAVR without SENTINEL 3.3%; p=0.662).
Secondary Analysis:
Secondary analyses of disabling stroke in the US cohort showed a statistically significant 73% relative risk reduction at discharge or 72-hours in TAVR with SENTINEL (0.4%) vs TAVR without SENTINEL (1.5%) (p=0.018).
No significant difference was observed in the OUS cohort (TAVR with SENTINEL 0.7% vs TAVR without SENTINEL 1.0%; p=0.545).
Study Design

Stroke Outcomes by Geographic Region

All-stroke and disabling stroke at discharge or 72 hrs after TAVR by geographic region
Ischemic Stroke Outcomes by Geographic Region

Ischemic Stroke at discharge or 72 hrs after TAVR by geographic region
In the US cohort, ischemic stroke rate was ~4-fold lower in TAVR with SENTINEL patients (0.3%) vs TAVR without SENTINEL (1.3%).
In OUS cohort, ischemic stroke showed a ~2-fold lower rate in TAVR with SENTINEL patients (0.5%) vs TAVR without SENTINEL (0.9%).
Conclusions
In US patients, TAVR with SENTINEL was associated with a 50% relative risk reduction for overall stroke and a 73% relative risk reduction for disabling stroke compared to TAVR without SENTINEL at discharge or 72 hrs.
A treatment effect on stroke risk reduction was not observed in OUS cohort.
Patients treated with SENTINEL and discharged within 72 hrs were more likely to be discharged home compared to patients who did not have SENTINEL used during TAVR.
For patients who did require a longer hospital stay (>72 hrs), those who did not have SENTINEL used were more likely to need higher-intensity care.
Lastly, there was no statistically significant difference between the two cohorts when looking at safety rates of all-cause mortality and stroke, acute kidney injury, or CEP access site-related vascular complications.
Regional differences in patient practices and procedural characteristics may impact the effectiveness of SENTINEL in reducing TAVR-related stroke.
STS/ACC TVT Registry
Impact of Cerebral Embolic Protection Devices on Disabling Stroke after TAVR: Results from the TVT Registry5
Study Objectives
- To describe updated temporal trends and site-level variability in use of SENTINEL among patients undergoing TAVR in the US.
- To use real-world data to investigate whether use of SENTINEL is associated with reduction in disabling stroke in patients undergoing TAVR in contemporary practice.
Study Design
General Design: Observational retrospective study of the relationship between use of SENTINEL and TAVR outcomes
Data Source: STS/ACC TVT Registry
Inclusion Criteria: First isolated Transfemoral TAVR between 1/2018 and 6/2023; includes all TAVR devices, bicuspid valve, valve-in-valve procedures
Exclusion Criteria: Emergent procedures; alternative access; sites performing <20 TAVR/yr; CEP other than SENTINEL
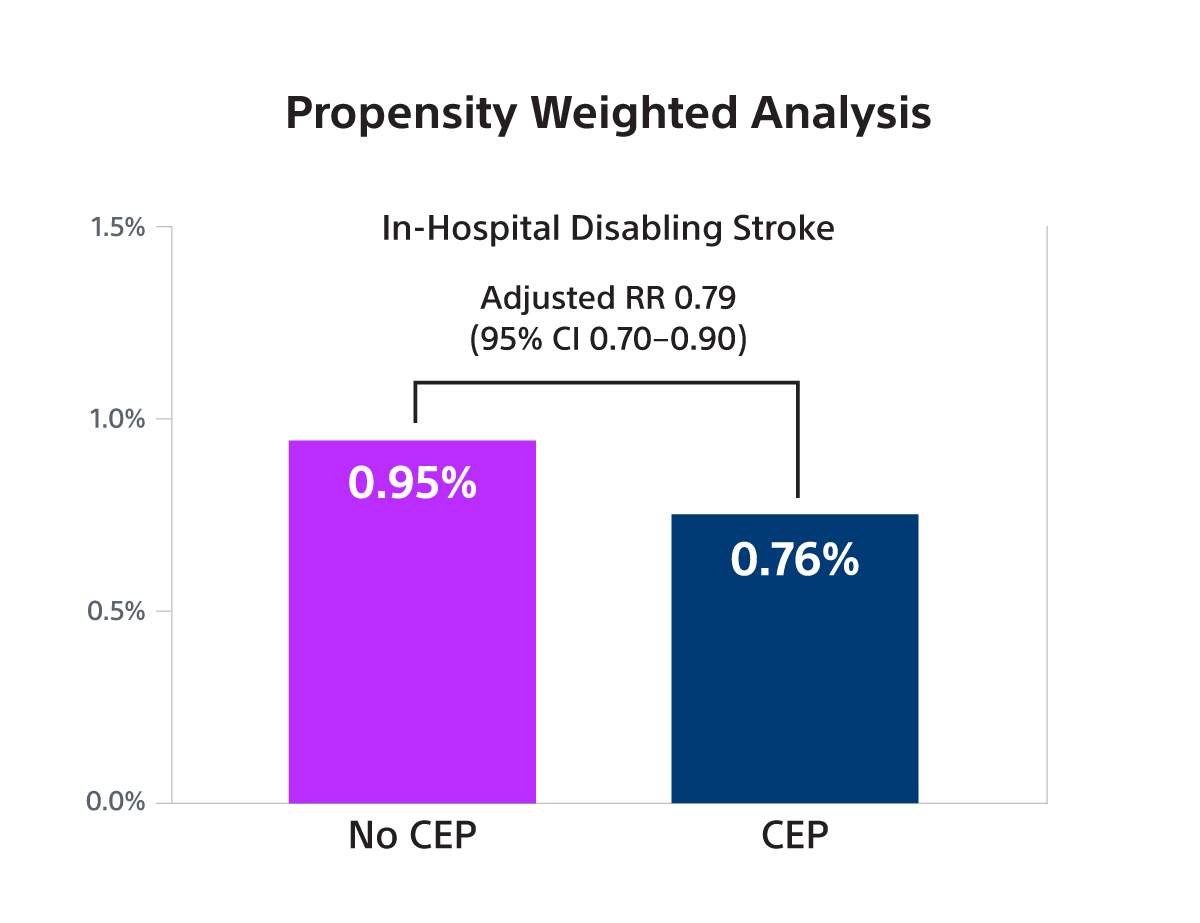
Primary Endpoint: In-hospital disabling stroke
Key Points
- The STS /ACC TVT Registry Stroke data were presented as a Late- Breaking Clinical trial at NY Valves on Wednesday, June 5, 2024 by Dr. David Cohen from St. Francis Hospital and Dr. Neel Butala from the Rocky Mountain Regional VA Medical Center, leaders of this Investigator- Sponsored Research (ISR) study.
- Results from a prior TVT Registry analysis4 (>120K TAVR patients), and from the PROTECTED-TAVR5 trial, examining the benefits of using SENTINEL during TAVR, generated inconclusive findings related to all-stroke. Therefore, the present study (observational, retrospective study), using the TVT registry was designed to investigate whether the use of SENTINEL is associated with reduction in disabling stroke in patients undergoing TAVR in contemporary practice.
- The STS/ACC TVT registry collects data on patients’ demographics, comorbidities, and outcomes in the US outside of clinical trials. The study population consisted of N=414,649 TAVR patients of whom 53,389 (12.9%) received a SENTINEL between 1/2018-6/2023. The primary outcome was in-hospital disabling stroke. A proxy definition of disabling stroke – defined as non-home discharge - was validated and used for the analysis.
Results
Analytic Approaches
Primary: Instrumental Variable Analysis
- Technique originally developed in economics that takes advantage of “natural experiments” to approximate randomization
- Under appropriate assumptions, can account for both measured and unmeasured confounding and support causal inference
- Instrument = site-level preference for CEP use during the calendar quarter
Secondary: Propensity Score Weighting
- Propensity score to predict CEP use developed based on 32 demographic, clinical, and hospital-level characteristics
- Risk-adjusted comparisons performed using overlap propensity weighting
Main Study Findings
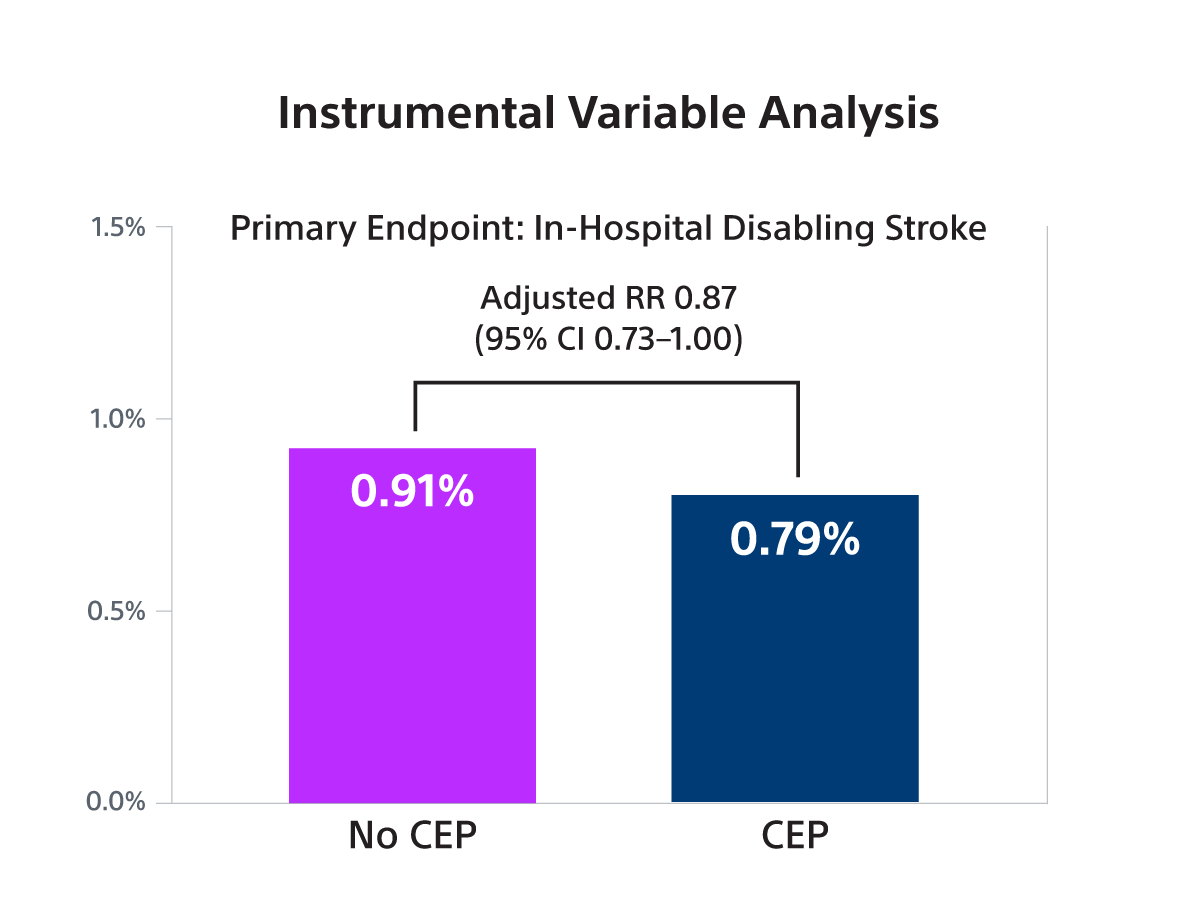
- To evaluate the association between SENTINEL use and disabling stroke, an instrumental variable analysis (IV) with site-level preference for SENTINEL use as the instrument was performed. In addition, a propensity score-based comparison (propensity weighting, PW) was performed as a secondary analysis.
- SENTINEL use was associated with a small, borderline significant reduction in disabling stroke in both IV analysis (RR 0.87; 95% CI: 0.73-1.00) and PW analysis (OR 0.79; 95% CI: 0.70-0.90) but was not associated with a reduction in non-disabling stroke.
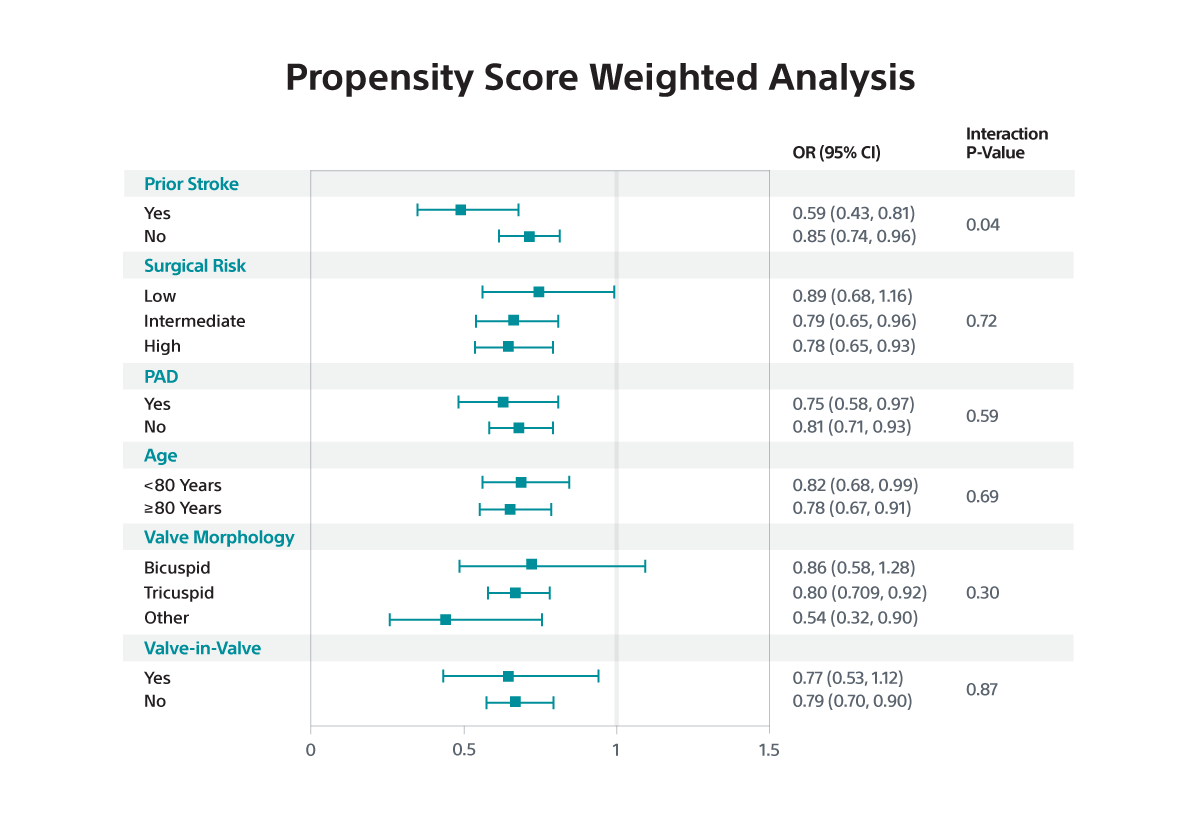
- In subgroup analyses, the benefit of SENTINEL was greater among patients with prior stroke versus those without (interaction p<0.05 for IV and PW).
Conclusions
- In this large observational study, it was found that SENTINEL use was associated with a small, borderline statistically significant reduction in the risk of stroke associated with death or a discharge to a non-home location (a proxy for disabling stroke) that was at the lower bound of what was observed in the PROTECTED-TAVR trial.
- The relative risk reduction in disabling stroke with SENTINEL use was amplified among the subset of patients with prior stroke.
- These findings provide evidence that supports a true clinical benefit of SENTINEL use for patients undergoing TAVR, limited to prevention of disabling stroke

The PROTECTED TAVR Study
Study objectives
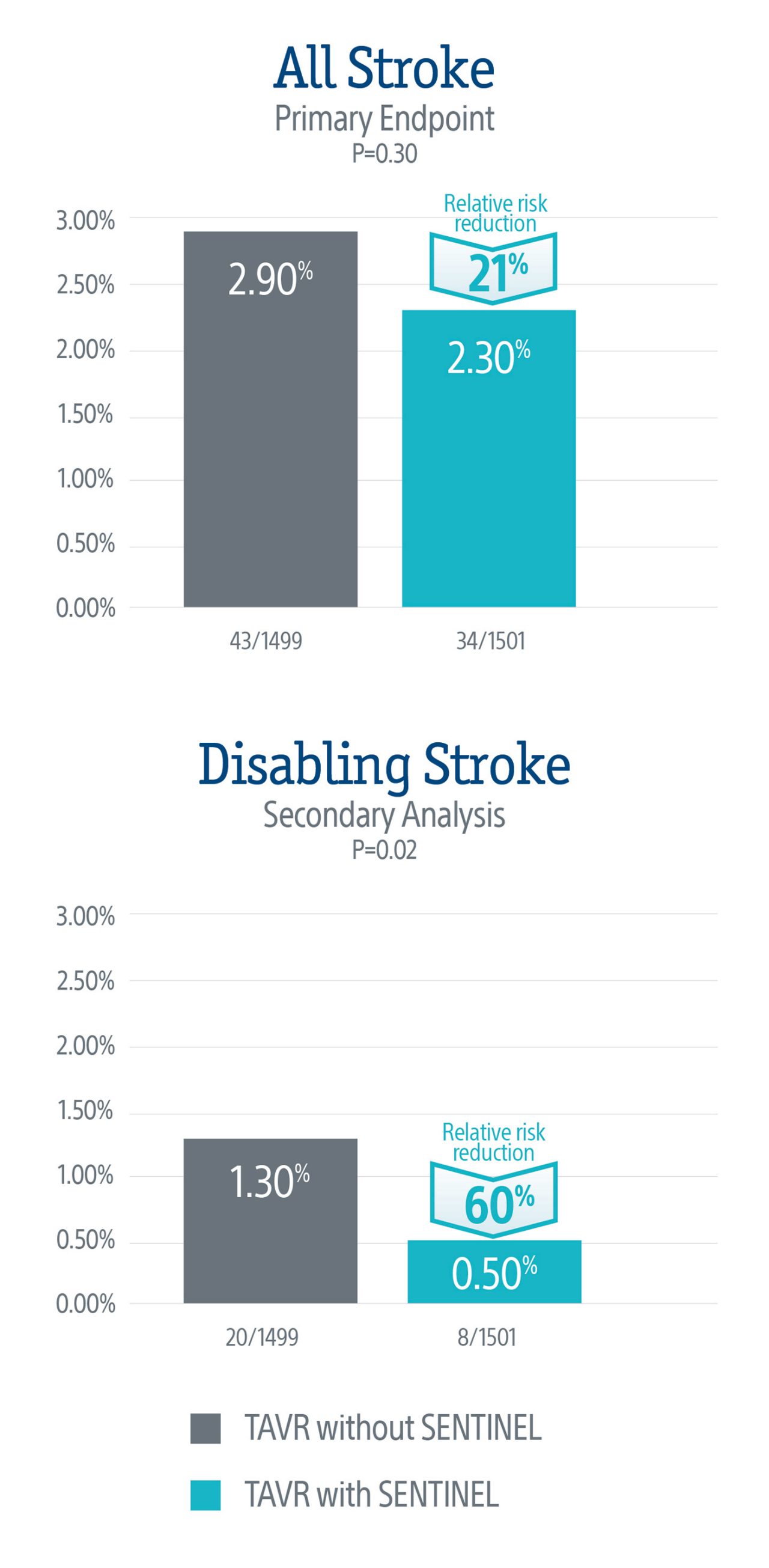
To demonstrate that use of the SENTINEL CPS significantly reduces the risk of peri-procedural stroke (≤ 72-hours) after TAVR.
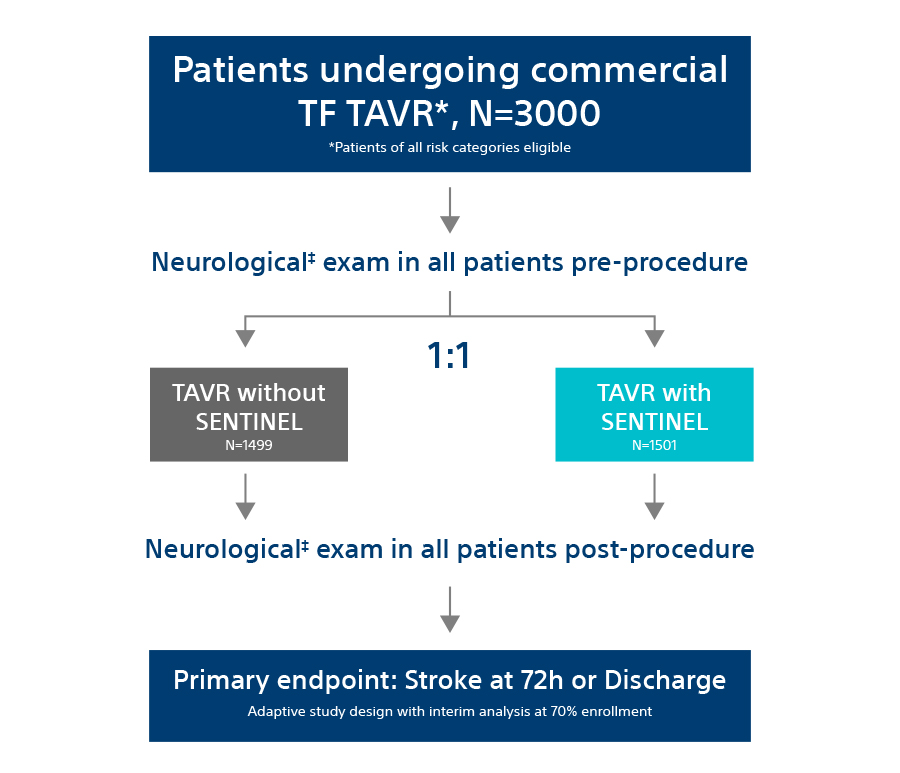
The PROTECTED TAVR Study is the largest randomized TAVR trial to date with 3,000 patients enrolled at >50 global sites who were randomized 1:1 – patients protected with SENTINEL vs. no use of SENTINEL during TAVR. All risk categories were eligible for inclusion, including low risk patients and all commercially available valves included as part of the trial.
Primary Endpoint: All Stroke (hemorrhagic, ischemic, or undetermined status; disabling or non-disabling) through 72-hours post TAVR procedure or hospital discharge.
Transient ischemic attack (TIA) and delirium were also reported on as part of the secondary neurological endpoints.
All patients enrolled in the trial underwent neurological examination at baseline and post TAVR procedure (discharge or 72-hours, whichever came first). This assessment was performed by a neurology professional (board certified/board eligible neurologist, neurology fellow, neurology physician assistant, or neurology nurse practitioner).
PROTECTED TAVR results
The primary endpoint did not meet statistical significance, but the data demonstrated a numerical trend towards a lower rate of stroke in patients treated with the SENTINEL device, representing a 21% relative risk reduction in all stroke through 72-hours or time of hospital discharge.
A secondary analysis demonstrated a statistically significant 60% relative risk reduction in disabling stroke through 72-hours or time of hospital discharge in patients treated with the SENTINEL device.