Clinical Data
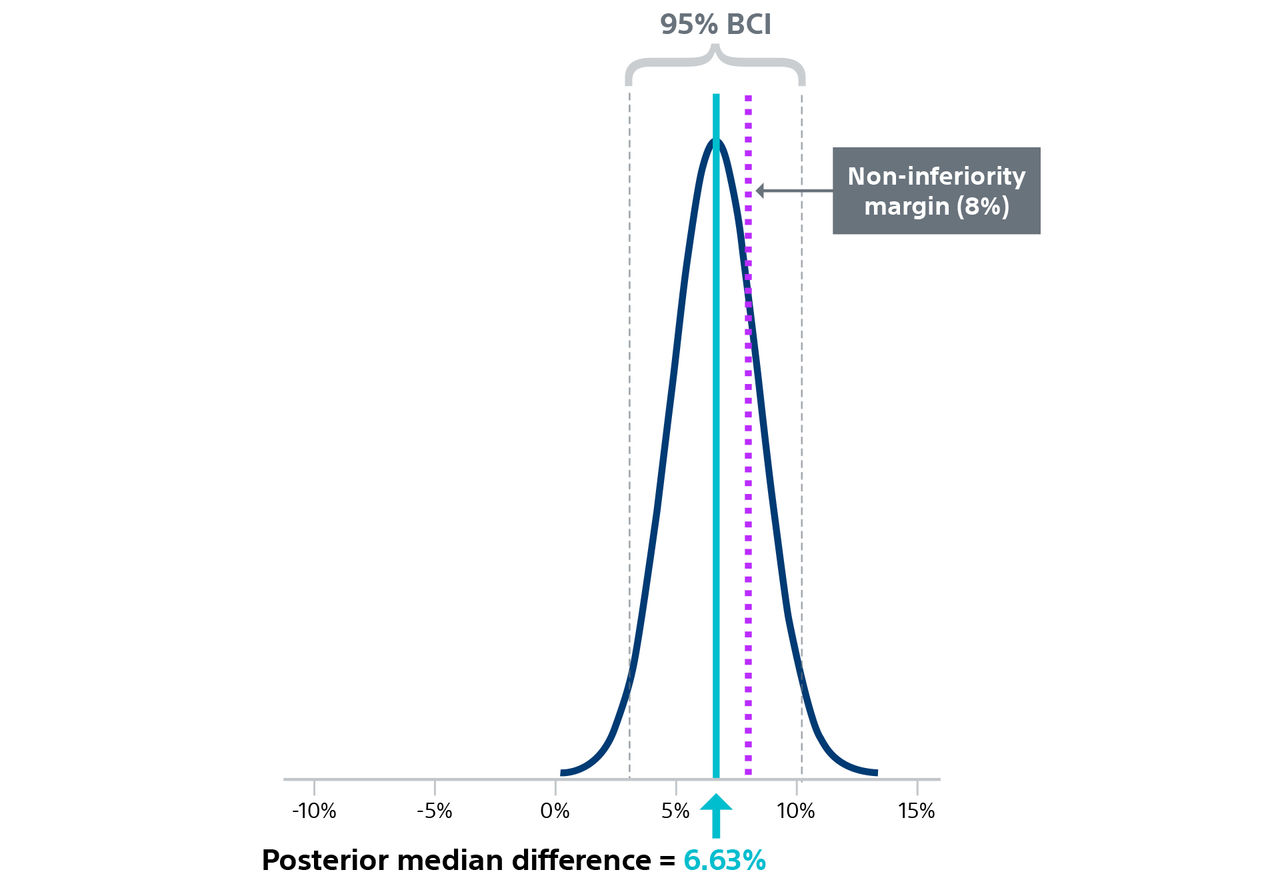
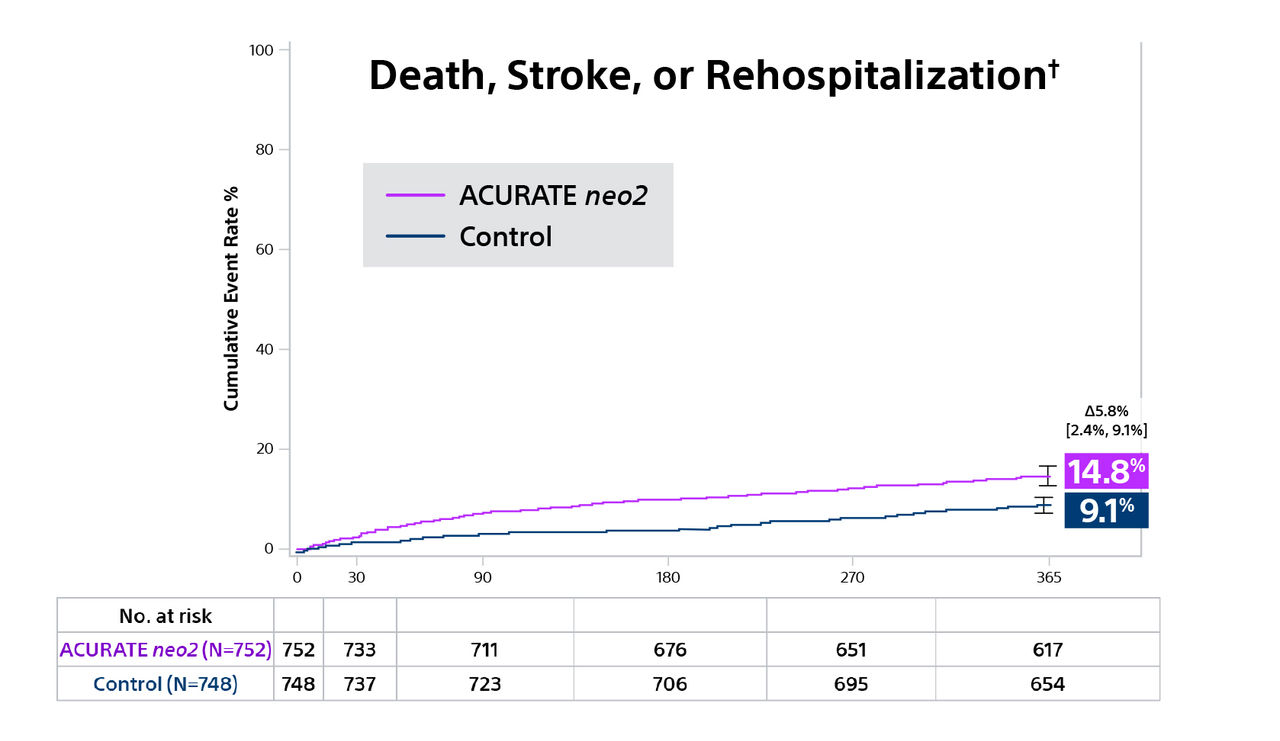
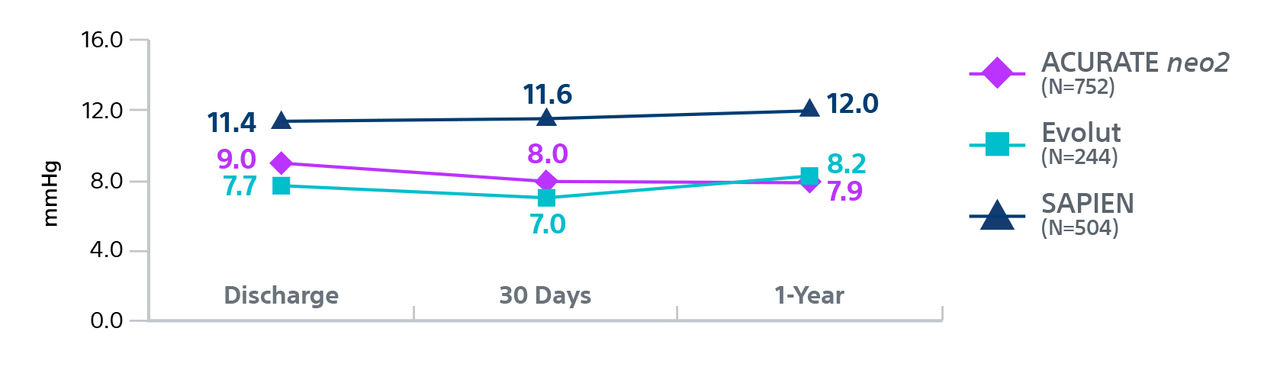
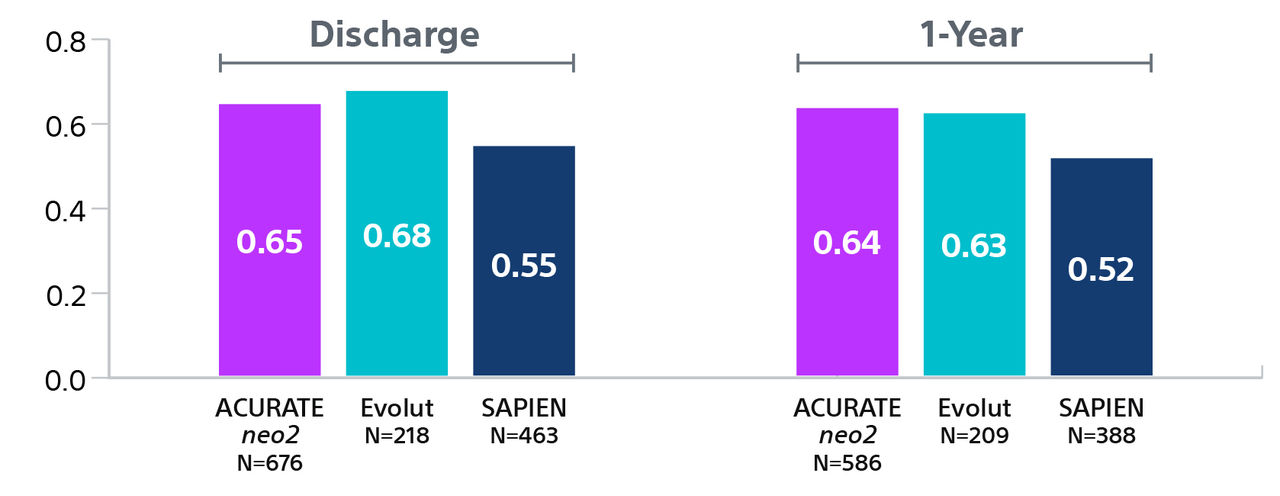
† Hospitalization for valve-related symptoms or worsening congestive heart failure (NYHA class III or IV); per VARC-2 definition
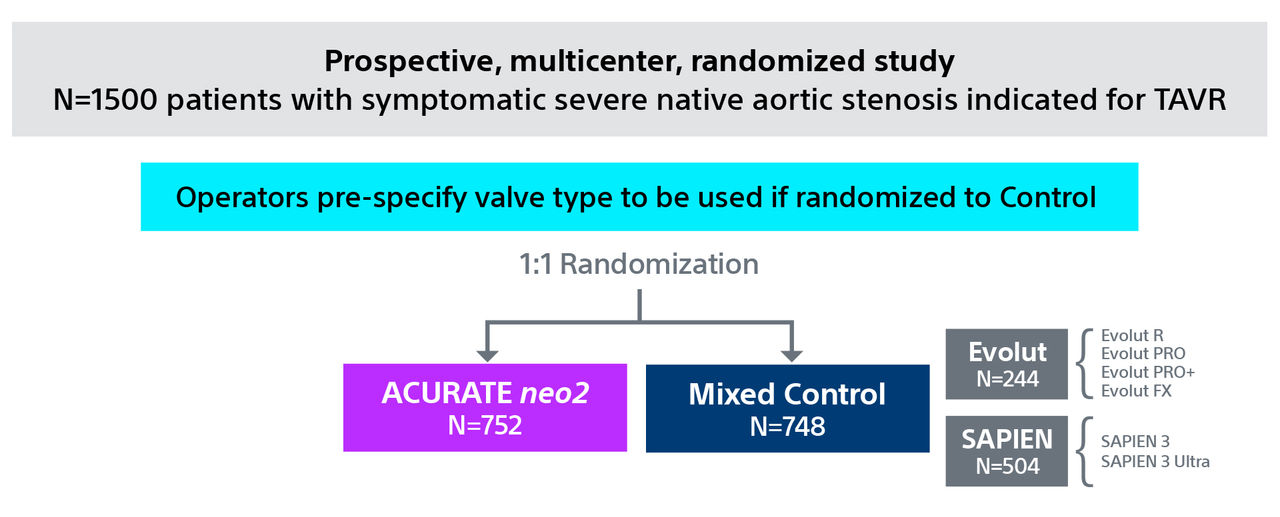
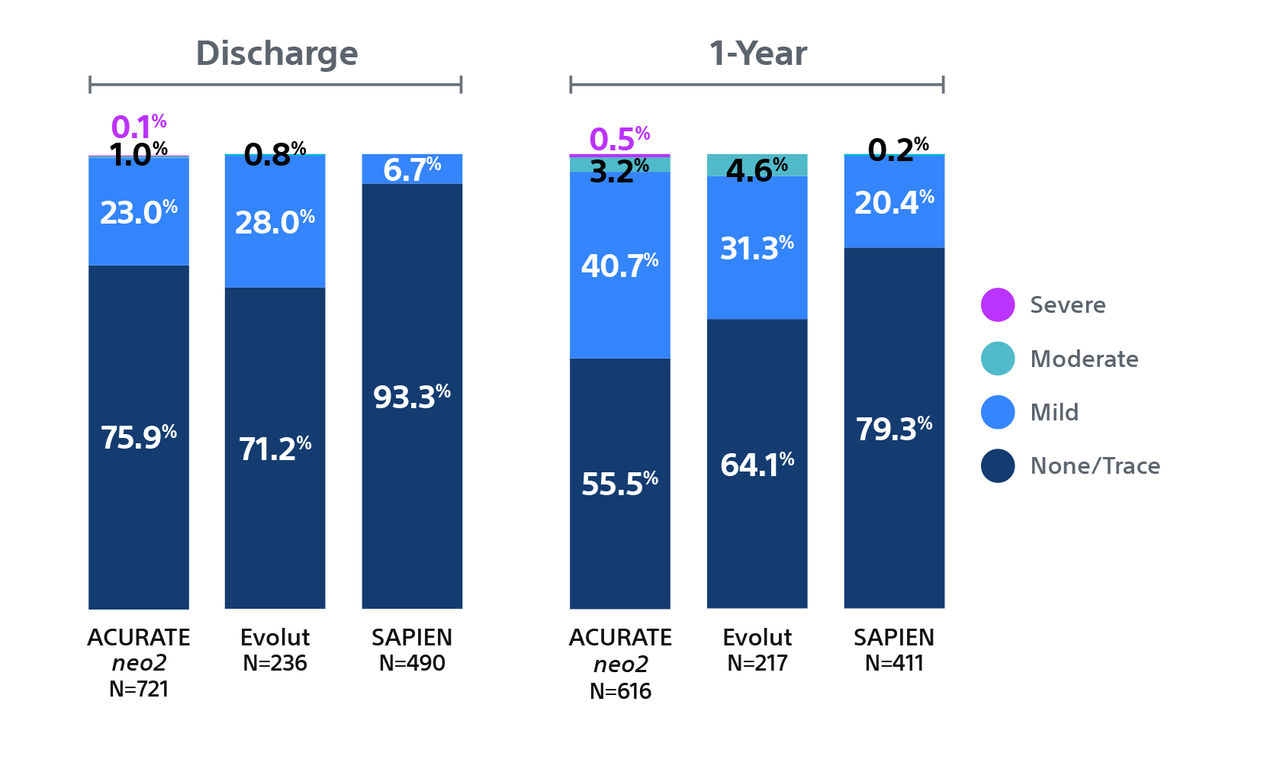
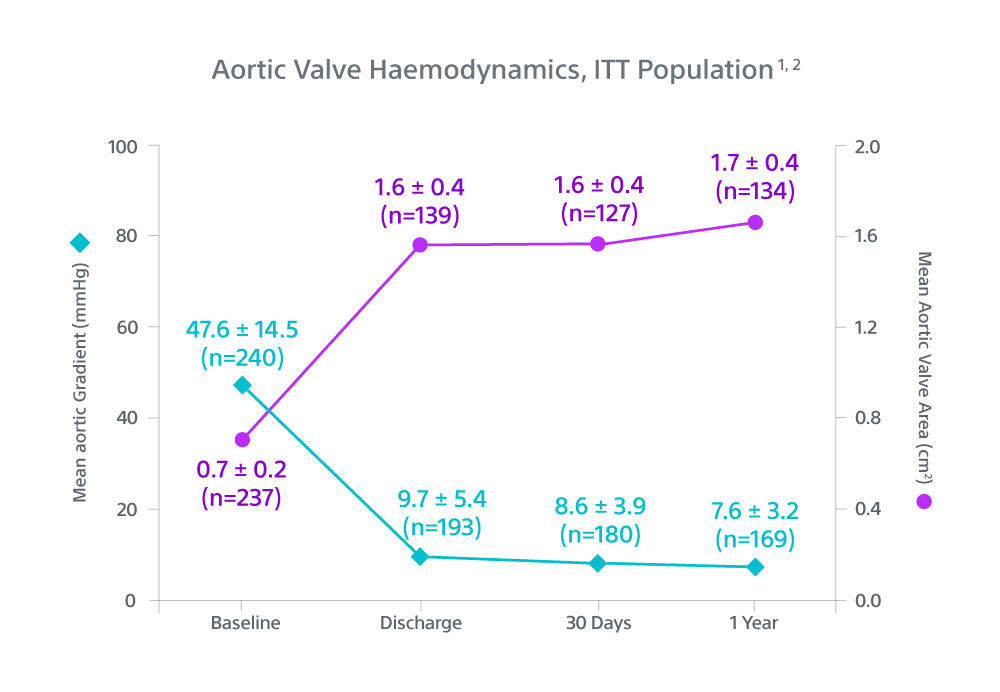
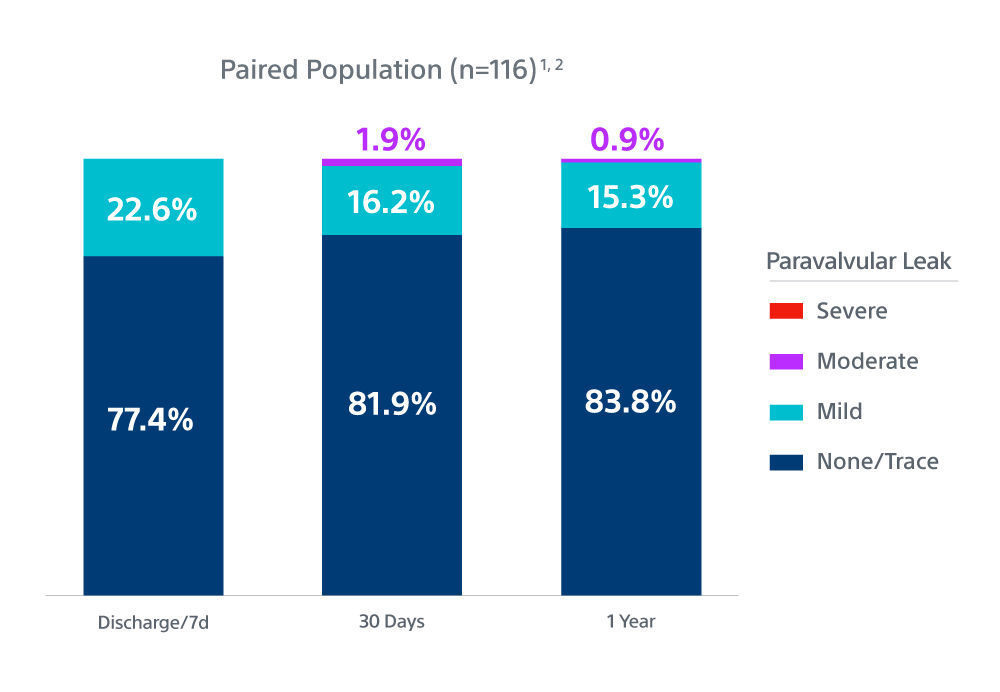
1. One-year Outcomes of ACURATE neo2 vs Approved TAVR Devices in All-risk Patients with Severe AS: the ACURATE IDE Trial, Presented by Dr Michael Reardon, TCT 2024.
Note: Control devices include Evolut™ R, Evolut™ PRO, Evolut™ PRO+, and Evolut™ FX and SAPIEN™ 3, SAPIEN™ 3 Ultra . All trademarks are the property of their respective owners.
ACURATE neo2 PMCF1 (N=250) 30-Day follow-up (N=246) | ACURATE neo2 PMCF2 (N=250) 1-yr follow-up (N=223) | |
| Mortality | 0.8% (0.8% CV) | 5.1% (3.4% CV) |
| All-Stroke | 0.8% (0% disabling) | 3.0% (1.3% disabling) |
| Rehospitalization | 0% | 1.7% |
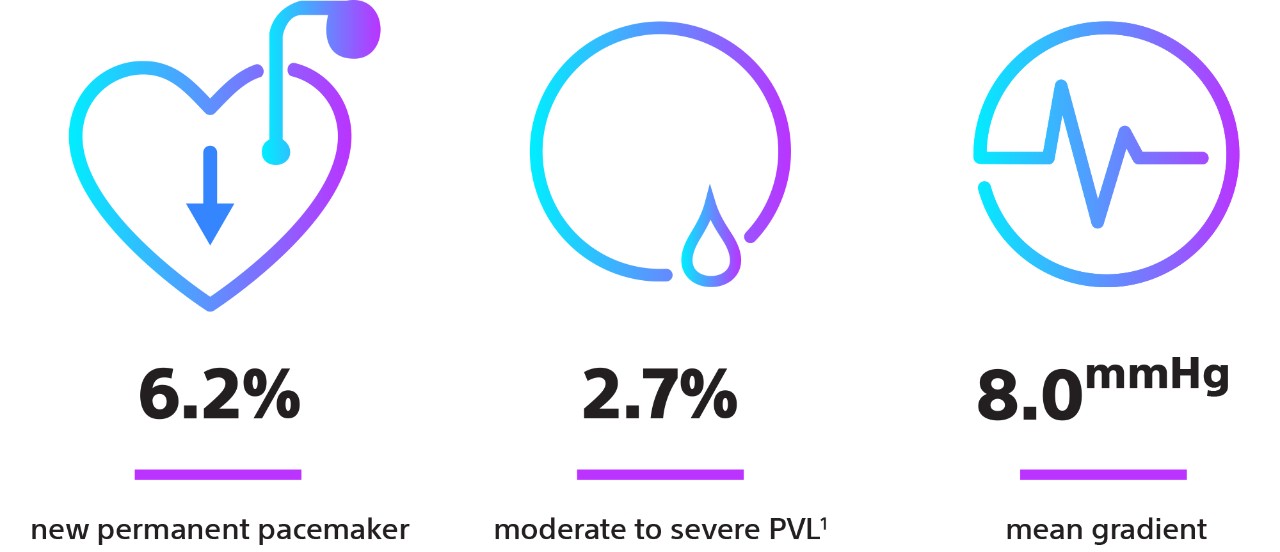
| New Permanent Pacemaker Rates | 6.1% | 7.8% |
| Mean AV Gradient | 8.6 mmHg | 7.6 mmHg |
| PVL ≥ Mod | 1.9% (0% severe) | 0.9% (0% severe) |
2. Kim WK, et al. EuroIntervention. 2024 Jan 1;20(1):85-94. doi: 10.4244/EIJ-D-23-00823.
Results from the Early neo2 registry with the ACURATE neo2 TAVI valve.
Background: ACURATE neo2 is a transcatheter aortic valve implantation system consisting of a self-expanding bioprosthetic valve with supra-annular leaflet position and featuring innovations to facilitate placement accuracy and reduce paravalvular regurgitation.
Methods and Results: The goal of the Early neo2 (Early neo2 Registry of the ACURATE neo2 TAVI Prosthesis) was to gather real-life data on safety and efficacy in a European transcatheter aortic valve implantation population treated with ACURATE neo2. Data were collected from 554 consecutive patients treated with ACURATE neo2 at 12 European sites (mean age, 82 years; 66% women; mean European System for Cardiac Operative Risk Evaluation II, 4.5%}3.8%) between September 2020 and March 2021. The composite primary end point was the occurrence of any of the following: postoperative (in-hospital) paravalvular regurgitation grade ≥2, in-hospital acute kidney injury stage 3, postoperative pacemaker implantation, 30-day death, and 30-day stroke. The primary end point occurred in 12.6% of patients. The 30-day rates for all-cause death and all stroke were 1.3% and 2.7%, respectively, and 1.5% of patients exhibited stage 3 acute kidney injury. A total of 34 patients (6.2%) received a postoperative permanent pacemaker. Per core laboratory–adjudicated echocardiographic analysis, mean postoperative aortic valve gradient was 7.6}3.3 mm Hg, and 2.8% of patients exhibited paravalvular regurgitation grade ≥2.
Conclusions: In this report of postmarket use of the ACURATE neo2 valve in a real-world transcatheter aortic valve implantation population, patients exhibited favorable postoperative hemodynamics and clinical outcomes and a low rate of postoperative pacemaker implantation.
Transcatheter Aortic Valve Replacement with Self-Expanding ACURATE neo2: Postprocedural Hemodynamic and Short-Term Clinical Outcomes.
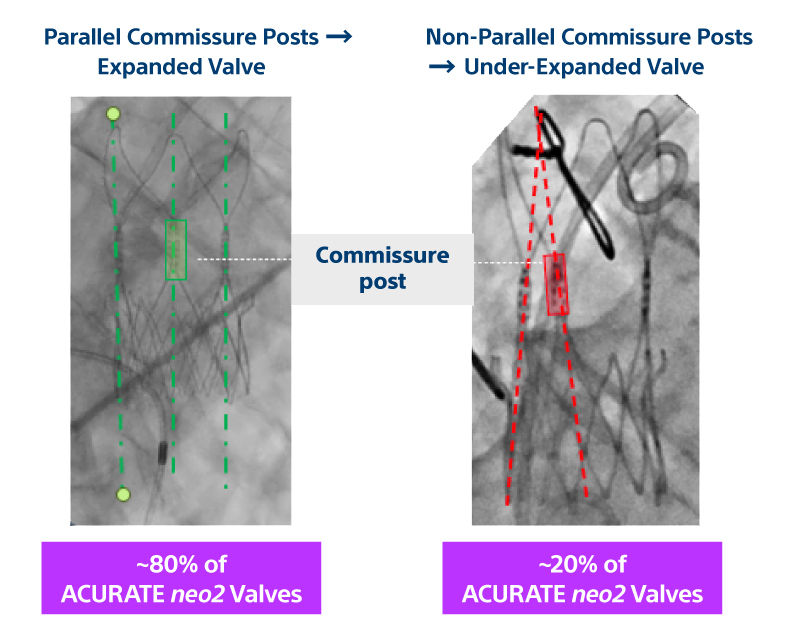
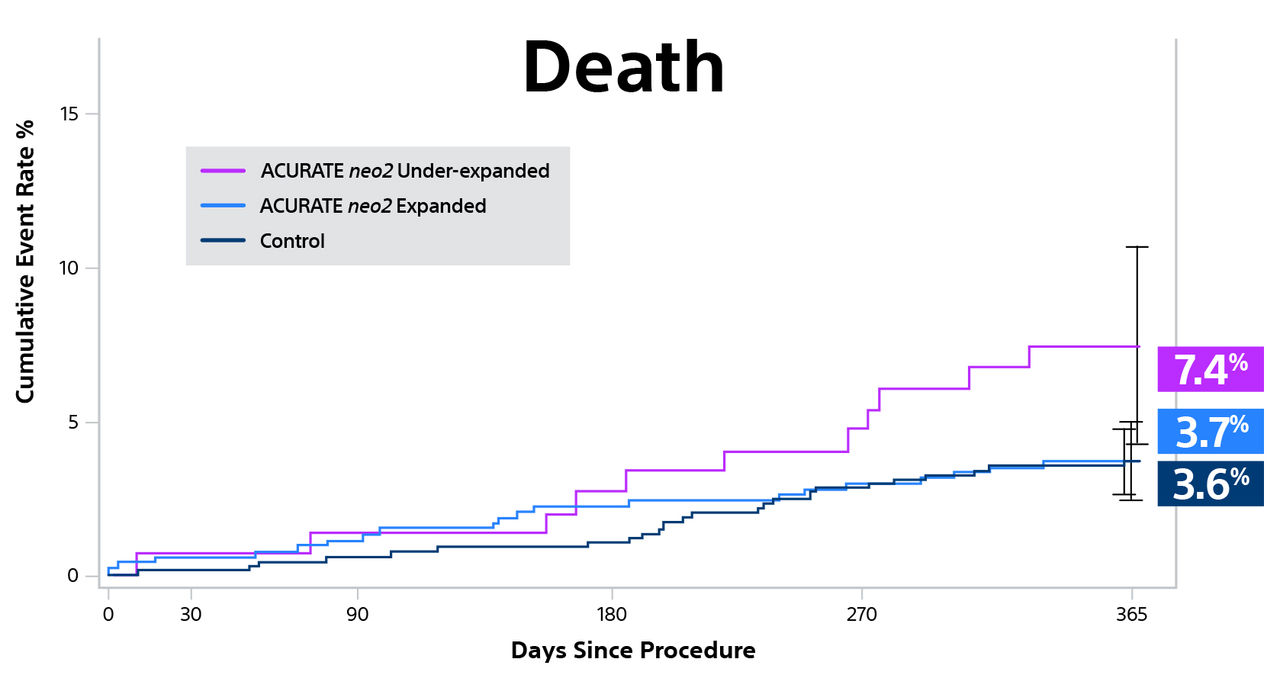
Background: The first-generation ACURATE neo transcatheter heart valve (THV) (Boston Scientific) was associated with a non-negligible occurrence of moderate or greater paravalvular aortic regurgitation (AR) following transcatheter aortic valve replacement. To overcome this issue, the ACURATE neo2 iteration, which incorporates a taller outer skirt aimed at reducing the occurrence of paravalvular AR, has recently been developed.
Objectives: The aim of this study was to assess the efficacy and safety of the ACURATE neo2 (Boston Scientific) THV in patients with severe aortic valve stenosis.
Methods: ITAL-neo was an observational, retrospective, multicenter registry enrolling consecutive patients with severe aortic valve stenosis, treated with first- and second-generation ACURATE neo THVs, via transfemoral and trans-subclavian access, in 13 Italian centers. One-to-one propensity score matching was applied to account for baseline characteristics unbalance. The primary endpoint was the occurrence of moderate or greater paravalvular AR on predischarge echocardiographic assessment. Secondary endpoints included postprocedural technical success and 90-day device success and safety.
Results: Among 900 patients included in the registry, 220 received the ACURATE neo2 THV, whereas 680 were treated with the first-generation device. A total of 410 patients were compared after 1:1 propensity score matching. The ACURATE neo2 THV was associated with a 3-fold lower frequency of postprocedural moderate or greater paravalvular AR (11.2% vs 3.5%; P < 0.001). No other hemodynamic differences were observed. Postprocedural technical success was similar between the 2 cohorts. Fewer adverse events were observed in patients treated with the ACURATE neo2 at 90 days.
Conclusions: Transfemoral transcatheter aortic valve replacement using the ACURATE neo2 was associated with a significant lower frequency of moderate or greater paravalvular AR compared with the earlier generation ACURATE neo device, with encouraging short-term safety and efficacy.
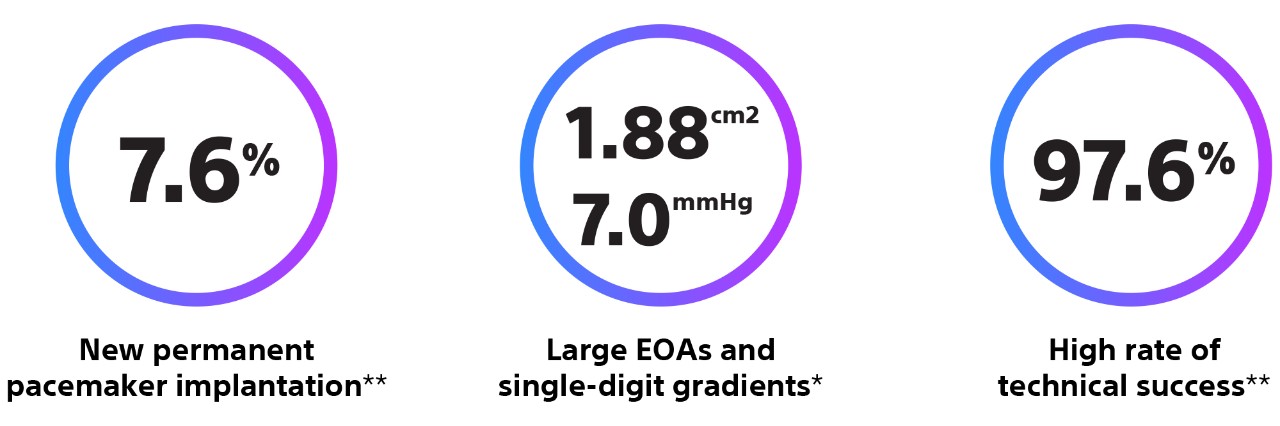
*Pre discharge.
**In hospital.Buono A, et al. JACC Cardiovasc Interv. 2022 Jun 13;15(11):1101-1110. doi: 10.1016/j.jcin.2022.02.027.
ACURATE neo2 versus SAPIEN 3 Ultra for transcatheter aortic valve implantation.
Background: No comparative data exist with the latest generation self-expanding ACURATE neo2 (Neo2) and the balloon-expandable SAPIEN 3 Ultra (Ultra) transcatheter heart valves (THV).
Aims: We aimed to compare the outcomes after transcatheter aortic valve implantation (TAVI) using the Neo2 and the Ultra THV.
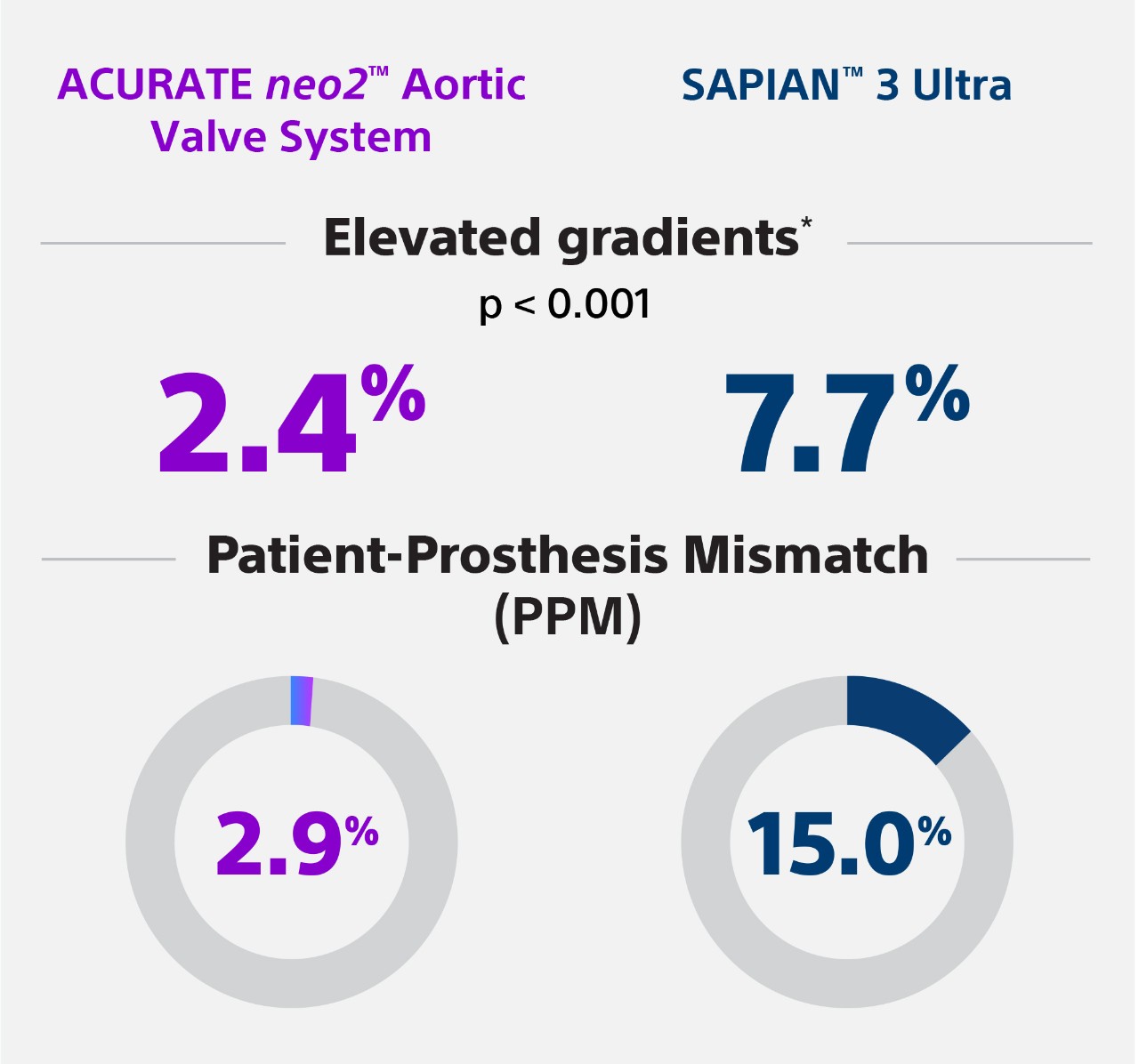
Methods: A total of 1,356 patients at 4 centres were treated either with the Neo2 (n=608) or the Ultra (n=748). The primary endpoint was device success according to the latest Valve Academic Research Consortium definitions. The association of the THV used and the primary endpoint was assessed using inverse probability treatment weighting (IPTW) and 1:1 propensity score matching (PSM), which identified 472 matched pairs. Results: After PSM, there were no relevant differences between the groups. While rates of moderate to severe paravalvular leakage (PVL) were overall low (0.6% vs 1.1%; p=0.725), elevated transvalvular gradients (≥20 mmHg) were less frequent with the Neo2 (2.4% vs 7.7%; p<0.001), which translated into a significantly higher rate of device success with the Neo2 compared with the Ultra (91.9% vs 85.0%; p<0.001). Consistently, the Neo2 was associated with higher rates of device success in the IPTW analysis (odds ratio [OR] 1.961, 95% confidence interval [CI]: 1.269-3.031; p=0.002). Rates of mild PVL were significantly lower with the Ultra compared with the Neo2 (20.0% vs 32.8%; p<0.001). Clinical events at 30 days were comparable between the 2 groups.
Conclusions: Short-term outcomes after TAVI using the Neo2 or Ultra THV were excellent and, overall, comparable. However, transvalvular gradients were lower with the Neo2, which translated into higher rates of device success. Rates of mild PVL were significantly lower with the Ultra THV.
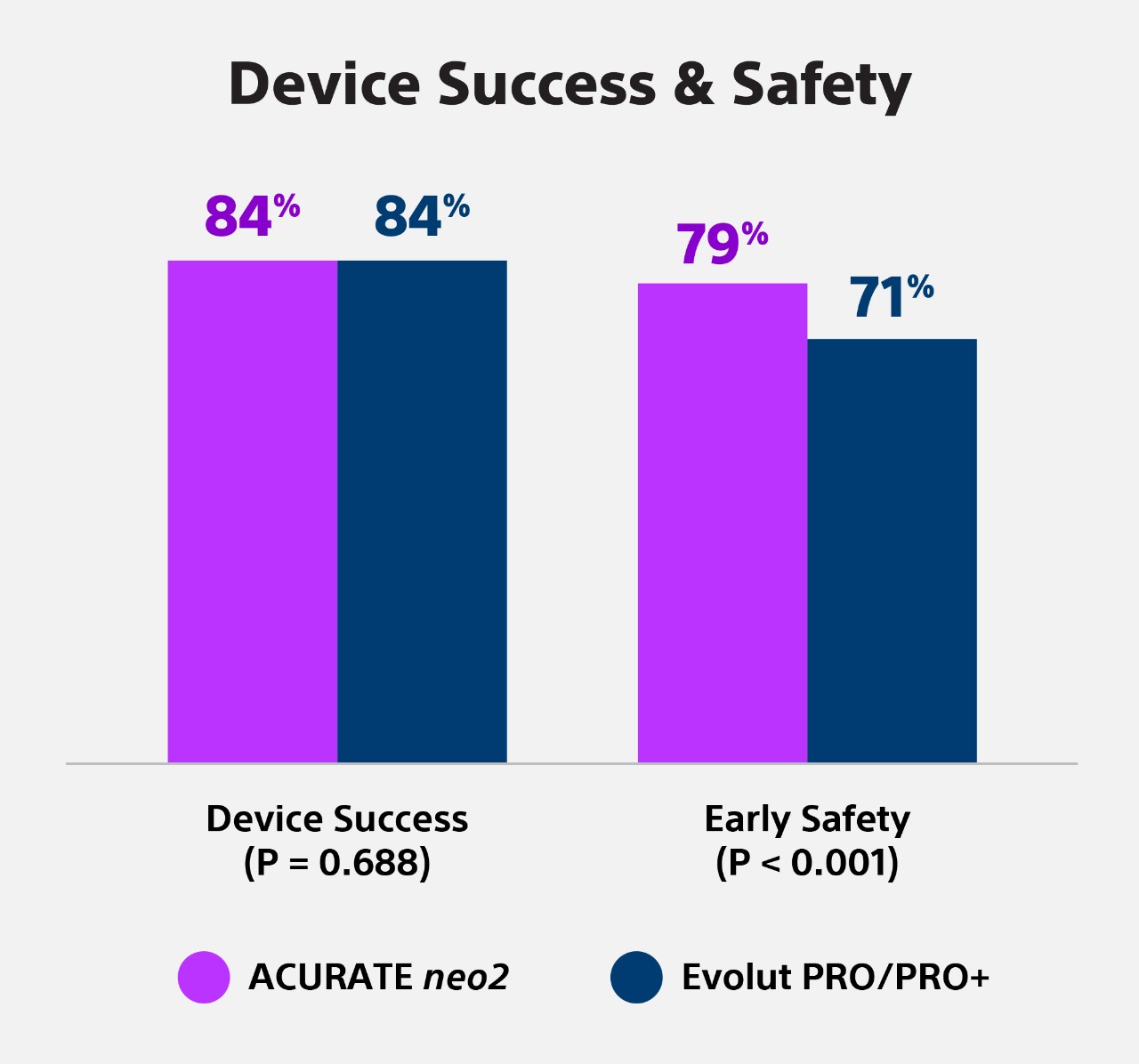
Comparison of transcatheter aortic valve replacement with the ACURATE neo2 versus Evolut PRO/PRO+ devices.
Background: The ACURATE neo2 (NEO2) and Evolut PRO/PRO+ (PRO) bioprostheses are new-generation self-expanding valves developed for transcatheter aortic valve replacement (TAVR).
Aims: We sought to compare the performance of the ACURATE neo2 and Evolut PRO/PRO+ devices.
Methods: The NEOPRO-2 registry retrospectively included patients who underwent TAVR for severe aortic stenosis with either the NEO2 or PRO devices between August 2017 and December 2021 at 20 centres. In-hospital and 30-day Valve Academic Research Consortium (VARC)-3 defined outcomes were evaluated. Propensity score (PS) matching and binary logistic regression were performed to adjust the treatment effect for PS quintiles. A subgroup analysis assessed the impact of aortic valve calcification.
Results: A total of 2,175 patients (NEO2: n=763; PRO: n=1,412) were included. The mean age was 82±6.2 years and the mean Society of Thoracic Surgeons score was 4.2%. Periprocedural complications were low, and both groups achieved high rates of technical success (93.1% vs 94.1%; p=0.361) and predischarge intended valve performance (96.0% vs 94.1%; p=0.056), both in the unmatched and matched analysis (452 pairs). Device success at 30 days was comparable (84.3% vs 83.6%; p=0.688), regardless of aortic valve calcification severity (p>0.05 for interaction). A suggestion for higher VARC-3 early safety in the NEO2 group was mainly driven by reduced rates of new permanent pacemaker implantation (7.7% vs 15.6%; p<0.001).
Conclusions: This retrospective analysis reports a similar short-term performance of the ACURATE neo2 platform compared with the new-generation Evolut PRO/PRO+ devices. Randomised studies are needed to confirm our exploratory findings.