Boston Scientific accounts are for healthcare professionals only.
Optimize your practice and help more patients
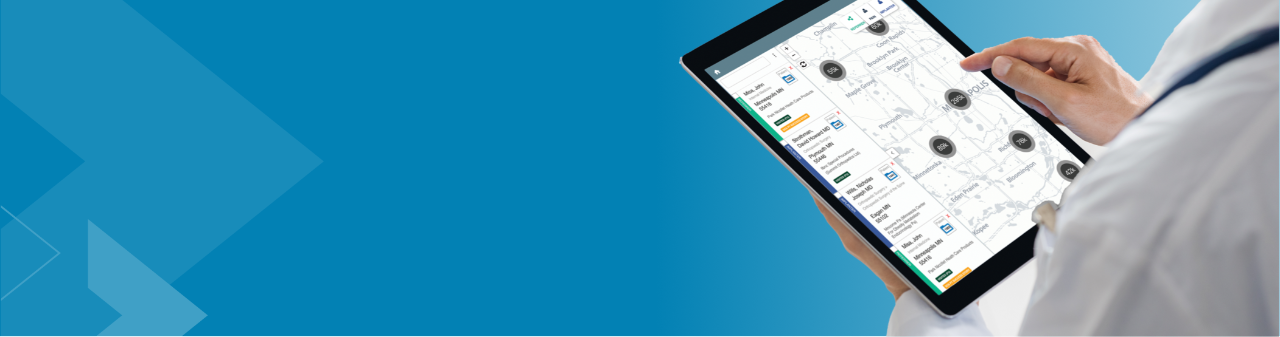
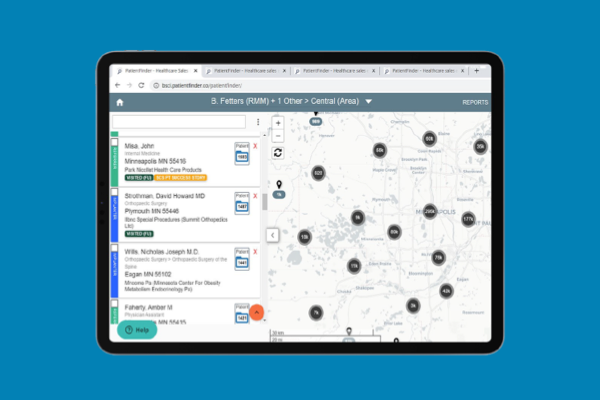
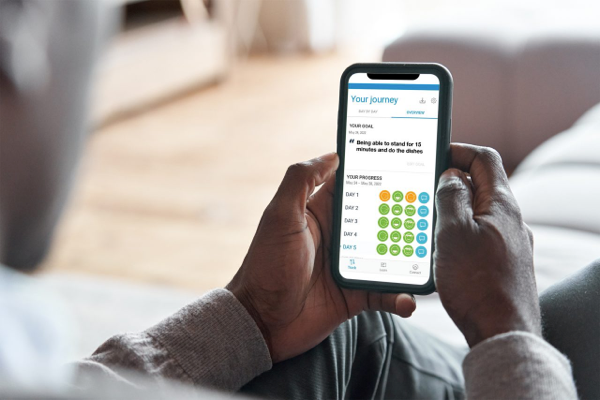
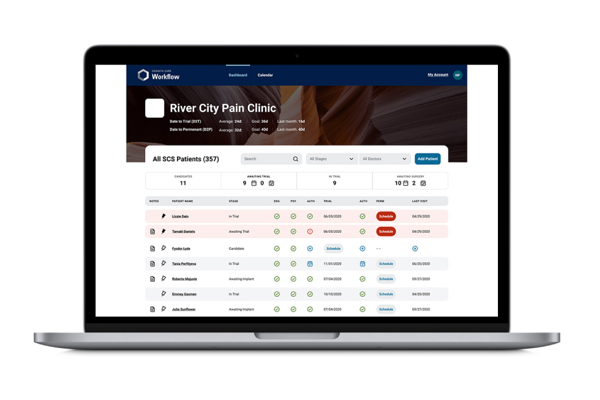
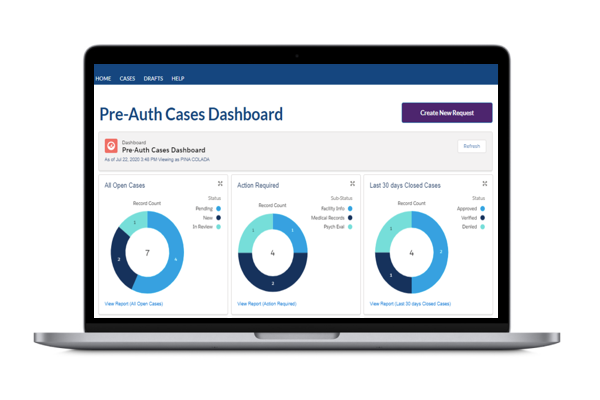
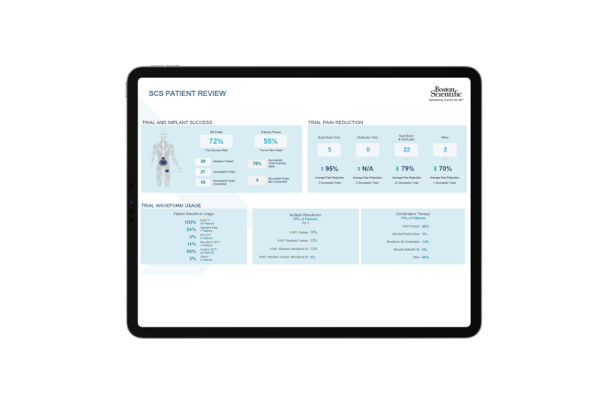
As a trusted partner in pain management, Boston Scientific created Cognita Practice Optimization to help you and your patients find success throughout the pain management journey. Our data-driven support solutions fuel customized insights to help you increase practice efficiency and set your patients up for success.
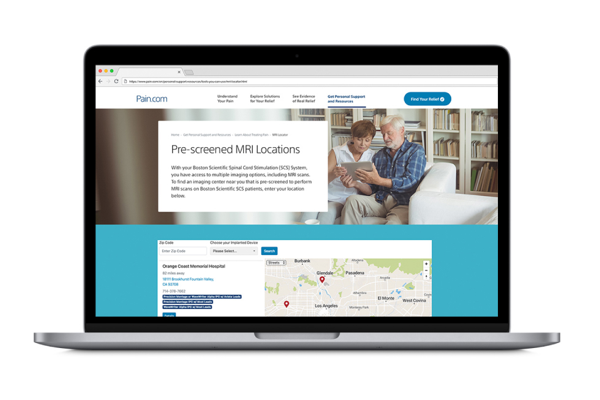
If you are a patient looking for effective pain solutions, visit pain.com.
See how Cognita Practice Optimization supports you and your patients through the pain management journey.

A comprehensive solution for you, your patients and your practice
Only 4 million out of 50 million pain patients will make it to a pain specialist1-2 and even fewer receive long-term durable pain relief. Why? The process is complex, and there are many challenges along the way. Cognita Practice Optimization supports physicians in a patient-centered approach to care. Using a combination of proprietary solutions, Cognita Practice Optimization delivers insights and tools that are tailored to each practice's unique needs. This allows you to identify more patients, effectively manage their care through your practice, and maintain success long-term.

Advancing success, maximizing patient outcomes
Your trusted partner in pain management
As a leader in pain management for over 20 years, Boston Scientific provides industry-leading solutions and support to physicians and patients worldwide.
A straight line to results
Experience Cognita Practice Optimization for your practice today.
A comprehensive pain portfolio

Discover WaveWriter Alpha™ SCS System

Learn about the Vertiflex™ Procedure†: Superion™ Indirect Decompression System

Access radiofrequency ablation for pain management education
References:
1. CDC Prevalence of Chronic Pain and High Impact Chronic Pain Among Adults – United States, 2016
2. 2018 Patient Finder local market data utilizing 154 pain-related diagnosis codes
*FAST MOA computational modeling by Dr. Warren Grill’s lab at Duke University. Gilbert et al., Computational modeling predicts dorsal columns are involved in fast-acting sub-perception spinal cord stimulation (SCS). SFN 2021.
†Superion™ Indirect Decompression System
SCS Indications for Use. The Boston Scientific Spinal Cord Stimulator Systems are indicated as an aid in the management of chronic intractable pain of the trunk and/or limbs including unilateral or bilateral pain associated with the following: failed back surgery syndrome, Complex Regional Pain Syndrome (CRPS) Types I and II, intractable low back pain and leg pain. Associated conditions and etiologies may be: radicular pain syndrome, radiculopathies resulting in pain secondary to failed back syndrome or herniated disc, epidural fibrosis, degenerative disc disease (herniated disc pain refractory to conservative and surgical interventions),arachnoiditis, multiple back surgeries. Contraindications, warnings, precautions, side effects. The SCS Systems are contraindicated for patients who: are unable to operate the SCS System, have failed trial stimulation by failing to receive effective pain relief, are poor surgical candidates, or are pregnant. Refer to the Instructions for Use provided with the SCS System or Pain.com for potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
IDS Indications for Use. The Superion™ Indirect Decompression System (IDS) is indicated to treat skeletally mature patients suffering from pain, numbness, and/or cramping in the legs (neurogenic intermittent claudication) secondary to a diagnosis of moderate degenerative lumbar spinal stenosis, with or without Grade 1 spondylolisthesis, having radiographic evidence of thickened ligamentum flavum, narrowed lateral recess, and/or central canal or foraminal narrowing. The Superion™ Interspinous Spacer is indicated for those patients with impaired physical function who experience relief in flexion from symptoms of leg/buttock/groin pain, with or without back pain, who have undergone at least 6 months of non-operative treatment. The Superion Interspinous Spacer may be implanted at one or two adjacent lumbar levels in patients in whom treatment is indicated at no more than two levels, from L1 to L5. Contraindications, warnings, precautions, side effects. The Superion Indirect Decompression System (IDS) is contraindicated for patients who: have spinal anatomy that prevent implantation of the device or cause the device to be unstable in situ (i.e., degenerative spondylolisthesis greater than grade 1), Cauda equina syndrome, or prior decompression or fusion at the index level. Refer to the Instructions for Use provided on www.vertiflex.com for additional Indications for Use, contraindications information and potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
RF Indications for Use: The Boston Scientific Radiofrequency Generators, associated Radiofrequency Lesion Probes and RF Cannula are indicated for use in procedures to create radiofrequency lesions for the treatment of pain or for lesioning only peripheral nerve tissue for functional neurosurgical procedures. The Boston Scientific RF Injection Electrodes are used for percutaneous nerve blocks with local anesthetic solution for radiofrequency lesioning of peripheral nerve tissue only. The Boston Scientific LCED and Stereotactic TCD Electrodes are indicated for use in radiofrequency (RF) heat lesioning of nervous tissue including the Central Nervous System.
Warnings: The Boston Scientific RF devices may cause interference with active devices such as neurostimulators, cardiac pacemakers, and defibrillators. Interference may affect the action of these active devices or may damage them. For appropriate guidance, consult the instructions for use for these active devices.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician